- Health
What do we know about the safety of bisphenol A in food packaging?

SUMMARY
Bisphenol A is an industrial chemical that has been used for decades to make certain plastics and resins. These materials are used as a coating in food and drink cans and reusable water bottles, from which small amounts of BPA can leach into foods and drinks. The use of BPA in food packaging has become a matter of intense debate because this chemical can act as a hormone in the body, potentially altering functions such as growth and reproduction. However, the U.S. Food and Drug Administration states that BPA in food is safe at the current levels of exposure in humans.
Introduction
Humans are exposed to many chemicals through the air, water, food, and the products we use in everyday life. While many of these chemicals are innocuous, others can pose a threat to human health depending on the amount or type of exposure. Some of these substances are particularly concerning because they can interfere with hormone functions (endocrine system) in the body, potentially leading to multiple health problems. These molecules are called endocrine disruptors.
One such molecule is bisphenol A (BPA), a synthetic chemical used to produce polycarbonate plastic and epoxy resin. These materials are present in the protective lining of food and drink containers, from which small amounts of BPA can leach into their content and be ingested by the consumer. This has led to recurrent online claims linking BPA to a variety of health problems, including metabolic and reproductive issues (examples here, here, and here).
Also, some users promote herbs or supplements that supposedly will help “detoxify” from BPA and prevent and treat its alleged harmful effects (examples here, here, and here). But no herb or supplement has been demonstrated in clinical studies to help eliminate BPA from the body or counter its alleged effects in humans. In fact, while mounting evidence from animal research indicates that even low levels of BPA can cause multiple health issues, whether and how current levels of BPA exposure impact people’s health is still a matter of debate.
In this article, we will explain the science behind the controversy regarding BPA safety and summarize current knowledge on the potential effects of BPA exposure on people’s health.
Why is BPA a cause of concern?
In 1993, researchers at Stanford University studying hormones found that BPA from plastic laboratory flasks could leach into the flask content, which interfered with their experiments by behaving like a hormone[1].
Indeed, BPA’s structure resembles that of estrogen (Figure 1), a natural hormone that helps develop and maintain female sex characteristics and has other essential functions in the body. This similarity enables BPA to bind to estrogen receptors in cells and induce changes in crucial estrogen-regulated body functions such as growth, development, reproduction, and metabolism.
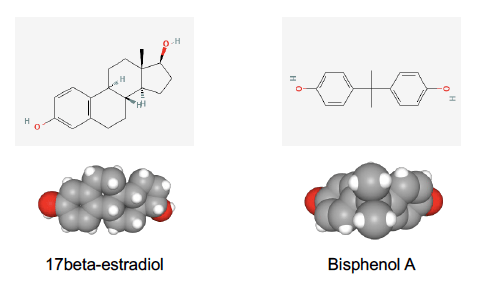
Figure 1. Bidimensional (up) and tridimensional (bottom) chemical structures of the estrogenic hormone estradiol, on the left, and bisphenol A, on the right. Source: PubChem.
Since the sixties, BPA has been incorporated into commonly used plastic products, including microwave-proof utensils, baby bottles and toys, dental composites and sealants, reusable water bottles, and canned foods. Later, multiple studies showed that trace amounts of BPA can migrate from the containers into food and drink, particularly when they contain acidic foods such as tomatoes, after long-term storage, and when the package is heated[2,3].
While ingestion through food and drink is a major route of exposure to BPA, it isn’t the only one. BPA can also be inhaled and absorbed through the skin through contact with plastic materials and thermal paper, commonly used for grocery receipts.
Confirming this widespread exposure, a U.S. National Health and Nutrition Examination Survey conducted between 2013 and 2014 found detectable levels of BPA in the urine of 95.7% of the 2,686 people aged six and older tested[4]. Other studies found BPA in human blood and sweat, breast milk, placenta, and umbilical cord blood, indicating that exposure to BPA starts already at the earliest stages of development[5-7].
The presence of small amounts of BPA in the body alone doesn’t necessarily imply that these levels are harmful in humans. However, such widespread and sustained exposure to an endocrine disruptor is a cause of concern. The main reason is that the body is sensitive to subtle changes in hormone signaling, which means that even low levels of BPA could be enough to alter the hormone balance and cause disease. The potential effects of BPA are particularly concerning during fetal and neonatal development, early childhood, and puberty, when the body is more susceptible to hormone changes[8,9].
Animal studies show multiple negative effects of BPA exposure
Hundreds of studies in rodents offer ample evidence that BPA can disrupt hormone signaling, reproduction, neurodevelopment, and metabolism, particularly during fetal and early developmental stages. Furthermore, many of these effects occur even at low BPA doses within the range of human exposure.
Some of the clearest effects of BPA in rodents appear in the reproductive system[10,11]. Several studies reported problems in fertility and ovarian, genital, and urinary functions in female mice exposed to BPA during development[12-14], sometimes with no other exposure to BPA than the amounts leaching from plastic animal cages and bottles[15]. In some studies, the animals showed increased testosterone levels and ovarian cysts, similar to what happens in women with polycystic ovarian syndrome [16,17], a condition in which endocrine disruptors are suspected contributors[16].
Likewise, male mice exposed to low levels of BPA either during development or in adulthood showed lower sperm counts and urinary problems compared to non-exposed mice[18-20].
Other studies suggest that BPA exposure might be a risk factor for certain types of cancer. For example, exposure to low doses of BPA during development resulted in prostate enlargement in male mice[21] and changes in the structure of the mammary glands and the endometrium—the tissue that lines the uterus—in female mice[14,17,22]. These changes are associated with a higher risk of prostate, breast, and endometrial tumors later in life[23,24].
Finally, developmental exposure to low levels of BPA affects rodent metabolism, causing changes in fat and glucose metabolism, liver function, and body weight[25,26]. A few studies also reported neurodevelopmental problems in rodents exposed to BPA during pregnancy and breastfeeding, particularly in the hippocampus, a brain area involved in memory and learning[27,28].
BPA exposure in humans correlates with multiple health problems, but the evidence is inconclusive so far
Despite overwhelming evidence showing that BPA is harmful in rodents at doses below 25 micrograms per kilogram of body weight per day, it remains unclear whether these doses are representative of human exposure and how the effects observed in animals translate to human health. Clinical trials provide the best quality evidence to evaluate the effects of a substance on human health. However, intentionally exposing people to a known endocrine disruptor whose effects in humans are unknown would be unethical.
Therefore, all evidence in humans comes from epidemiological studies that mostly correlate BPA measurements in urine and blood with observed differences in fertility, brain development, metabolism, cardiovascular function, and other conditions[29]. Jodi Flaws, a professor of comparative biosciences at the University of Illinois, and Linda Birnbaum, a former director of the National Institute for Environmental Health Sciences (NIEHS) as well as the National Toxicology Program (NTP), said in an email to Health Feedback that studies in humans link BPA to many adverse health effects, particularly reproductive issues in women.
The potential effect of BPA exposure during pregnancy and child development has received much attention, given the greater impact that endocrine disruptors can have during these periods. A few studies found that pregnant women who had recurrent miscarriages and those who delivered prematurely had higher urinary concentrations of BPA compared to women with no history of these issues[30]. Higher urinary BPA concentrations were also associated with an increased risk of the infant having low weight at birth[31].
Two U.S. studies suggested that the potential effects of BPA exposure during pregnancy might go beyond pregnancy outcomes and impact children’s behavior later on. These studies found that higher BPA concentrations in the urine of pregnant women correlated with more anxious and depressive behavior, poorer emotional control, and hyperactive behavior in their children three and seven years after birth[32,33].
Another reported correlation is between higher BPA concentrations and metabolic problems, including obesity and diabetes. For example, U.S. children and adolescents with obesity had high BPA levels in their urine[34,35], an association also observed in adults[36]. Also in adults, higher BPA urinary concentrations are associated with a higher risk of diabetes[36-40], high blood pressure, and cardiovascular disease[41-44].
Multiple studies found that high levels of BPA correlate with reproductive issues, especially in women[10]. Among women undergoing in vitro fertilization, those with higher concentrations of BPA in their urine and blood showed reduced ovarian function and fertility compared to women with lower BPA levels[45-49]. In men, BPA exposure was associated with increased testosterone concentrations in blood and reduced sperm quality[50,51].
While these studies help identify potential effects of BPA on human health, it is important to keep in mind that correlation alone isn’t enough to prove causation. Observing a correlation between two variables is a necessary factor to demonstrate a cause and effect, but it doesn’t necessarily imply that changes in one of them—in our case, BPA levels—caused the changes in the other one—in this case, health problems. Therefore, epidemiological studies alone can’t conclusively determine whether BPA caused or was the main contributor to the observed effects in humans.
Genetics and lifestyle factors such as smoking, diet, stress, and healthcare access in general and during pregnancy strongly influence overall health and can confound the interpretation of the results. For example, diets rich in ultra-processed foods expose people to higher levels of BPA from the packaging than diets based on fresh foods. But processed foods are associated to a higher risk of obesity and other conditions independently of the effect of BPA. In addition, people are exposed to hundreds of environmental chemicals, including many known and potential endocrine disruptors, that can also interact with each other[52,53].
Another limitation of the studies in humans is that urine measurements don’t necessarily reflect actual exposure to BPA. For example, most urine tests don’t measure BPA itself but a breakdown product formed during BPA elimination. This process depends not only on BPA levels in the body but also on the person’s metabolism, which varies individually and depending on gender and age[54]. Therefore, measuring urine concentration might lead to inaccurate estimations of human exposure to BPA.
Finally, some studies on BPA safety in animals and humans have produced conflicting results, showing no effects of BPA or inconsistencies among populations, age ranges, or gender[55-59]. For example, one study in rats conducted at Brown University and the U.S. National Center for Toxicological Research found “little, if any, impact on the testes”, contrary to earlier reports[53]. Another study from the School of Public Health at the University of California reported that higher urinary BPA concentrations were associated with increased overweight and obesity in nine-year-olds but not in five-year-olds. In contrast, increasing BPA concentrations in mothers during pregnancy were associated with decreased body fat among their daughters at nine years of age, although not among their sons[54].
These inconsistencies might be in part due to the fact that the effects of endocrine disruptors can be subtle and difficult to identify. In addition, the differences in study designs in terms of population characteristics, type of exposure, method of detection of BPA, and measured outcomes make them difficult to compare.
The takeaway is that we still don’t know how current levels of BPA exposure impact human health. This doesn’t necessarily mean that BPA exposure is safe but rather that we need more research to draw definite conclusions. In the meantime, evidence from experimental animals shows reasons for concern, and many researchers and advocacy groups have taken a “better safe than sorry” approach and called regulators to put stricter limits on BPA.
The disagreement between academics and regulators
In 2008, the U.S. National Toxicology Program’s (NTP) Center for the Evaluation of Risks to Human Reproduction published a report evaluating the potential reproductive and developmental effects of BPA in humans. The report concluded that BPA exposure was unlikely to cause reproductive effects in adults and “fetal or neonatal mortality, birth defects, or reduced birth weight and growth in their offspring”. However, it expressed “some concern for effects on the brain, behavior, and prostate gland in fetuses, infants, and children at current human exposures” due to BPA exposure from baby bottles and infant cups.
Following the report, several countries, including Canada, Denmark, France, and China, took action and banned the use of BPA in baby bottles. In 2011, the European Union also banned the sale of baby bottles containing BPA in all its member countries. France took one step further in 2015 and completely banned the use of BPA in food contact materials. The U.S. Food and Drug Administration (FDA) followed suit and officially banned BPA from use in baby bottles and drinking cups in 2012 but continues to state that “BPA is safe at the current levels occurring in foods”.
Much of the controversy about BPA safety lies in the fact that we still don’t know what is the safe level of exposure, if any, for humans. BPA is removed from the body via urine within 24 hours, and no evidence indicates it accumulates in humans. Based on this, a 2018 safety assessment by the FDA established the limit of BPA safety at five milligrams per kilogram of body weight per day, which is the lowest level observed to cause acute toxicity in rodents (LOAEL). The agency estimated that most people are exposed to a much lower amount, ranging between 0.2 to 0.5 micrograms (one-thousandth of a milligram) per kilogram of body weight per day.
However, many researchers and professional societies like the Endocrine Society and the European Society of Endocrinology[60] believe that the FDA regulation falls short and find the current animal and epidemiological evidence compelling enough to suspect that BPA causes or contributes to various health problems, even within the established safety levels[61].
An article in Science explained that this discrepancy is mostly rooted in the so-called guideline studies that regulators rely on for assessing chemical safety. These studies must follow good laboratory practice (GLP) standards, a federal rule that establishes the type of tests that are considered acceptable in toxicological studies and specify how to collect and analyze data. This rule was developed in the late seventies to avoid misconduct by industry-funded research following a scandal involving an American laboratory that falsified safety data on drugs, pesticides, and food additives in order to obtain regulatory approval.
Since then, agencies have relied on GLP studies for regulatory purposes. But many academic and government researchers warn that GLP compliance doesn’t necessarily guarantee the quality of the research and instead may introduce biases in regulatory decisions by prioritizing a few industry-funded studies over more extensive and scientifically rigorous academic research[62]. According to many researchers, industry-funded GLP studies are often not reproducible by independent laboratories, and many use out-of-date methods that aren’t sensitive enough to detect low-dose BPA effects.
To overcome these discrepancies and study “the full range of potential health effects from exposure to BPA”, the National Institute of Environmental Health Sciences, together with the NTP and the FDA, developed the Consortium Linking Academic and Regulatory Insights on BPA toxicity (CLARITY-BPA). The program involved a two-year core study in rats conducted by researchers at the FDA’s National Center for Toxicological Research (NCTR) according to GLP standards. Additionally, academic institutions conducted a series of complementary studies using blinded tissue samples or study animals from the NCTR.
The CLARITY-BPA consortium was a groundbreaking collaborative effort that improved our understanding of the effect of low BPA doses on health. However, it didn’t settle the debate between regulators and academics. In 2018, the consortium published the core study findings, mostly negative or inconclusive. The few effects observed were caused by BPA exposures higher than those relevant to humans, and the report concluded that BPA had “minimal effects” on rodents. But many of the independent researchers who conducted the complementary studies did report adverse effects at low-dose BPA with potential implications for humans[63,64].
In a 2015 assessment, the European Safety Authority (EFSA) concluded that consumer’s exposure to BPA was too low to cause harm and set a temporary tolerable daily intake of four micrograms per kilogram of body weight per day. Unlike the FDA, the EFSA didn’t base this maximum on the LOAEL but on the NOAEL, which is highest BPA dose that has been reported to have no harmful health effects on people or animals.
But following a new assessment in 2021, the agency proposed lowering this limit to 0.04 nanograms (one-millionth of a milligram) per kilogram of body weight per day. The EFSA concluded that the average exposure to BPA in the diet exceeded the tolerable daily intake in all age groups, indicating potential health concerns.
The EFSA report prompted several scientists and advocacy groups to petition the FDA to restrict the use of BPA in food packaging. As a result, in June 2021, the FDA agreed to reconsider the levels of BPA that can be considered safe, according to a press release from the Environmental Defense Fund.
On 19 April 2023, the EFSA published a re-evaluation of BPA’s safety aimed at filling some of the “data gaps and uncertainties” identified in its 2015 assessment, using published evidence that hadn’t been included in previous assessments. In the new assessment, the agency evaluated the potential effects of BPA on mammary gland proliferation, development, reproduction, metabolism, neurobehaviour, metabolism, and immune system, as well as evidence on DNA toxicity.
The EFSA concluded that it is “Unlikely to Very Unlikely” that BPA causes direct DNA damage. However, the evaluation identified health concerns regarding the effect of BPA on the immune system. Claude Lambré, chair of EFSA’s Panel on Food Contact Materials, Enzymes and Processing, explained in a press release:
“In the studies, we observed an increase in the percentage of a type of white blood cell, called T helper, in the spleen. They play a key role in our cellular immune mechanisms and an increase of this kind could lead to the development of allergic lung inflammation and autoimmune disorders”
Based on these results, the EFSA concluded that “bisphenol A in food is a health risk” and reduced again BPA’s tolerable daily intake to 0.2 nanograms per kilogram of body weight per day. This is 20,000 times lower than the previous temporary limit of four micrograms per kilogram of body weight per day, and hundreds to thousands of times lower than the 2015 EFSA estimates of dietary exposure to BPA.
Can we avoid the potentially harmful effects of BPA?
Some users claim that certain herbs like oregano can “detoxify” the body from BPA, and supplements like shilajit counter its effects by increasing testosterone levels. Shilajit is a dark matter that forms from plant decomposition in rocks at very high altitudes, like the Himalayan mountains. Despite being touted as a remedy for a variety of medical conditions, there is little evidence supporting most of these uses.
Preliminary studies in cells and animals suggest that shilajit might boost energy, increase testosterone levels, and improve cognition and heart health, but human research is very limited. While two small clinical trials observed that shilajit increased testosterone levels and improved sperm count and motility in men, the number of participants was too small to draw definite conclusions. These results would need to be replicated in independent, larger trials to confirm these effects.
Several dietary supplements, including antioxidants, iodine, and folic acid, are being investigated to help counter the adverse effects of endocrine disruptors[65]. But so far, no herb or supplement has been demonstrated to eliminate BPA from the body or mitigate its potential effects in humans, contrary to online claims.
Instead, toxicologists and environmental health experts recommend reducing BPA exposure by avoiding plastic containers, processed foods, and canned foods and drinks and choosing fresh foods and glass or stainless-steel containers rather than plastic ones. Research shows that these simple interventions effectively reduce exposure to BPA and other endocrine disruptors[65].
When using plastic containers, experts recommend not to put them in the microwave or dishwasher—because heat causes them to leach more BPA— and to throw them away when they look aged or damaged.
While manufacturers have replaced BPA in many plastic products with other alternatives, these replacements might not be a solution. As two articles in Science and National Geographic explained, the latest research suggests that many common BPA substitutes have chemical structures similar to BPA, which means that they may also exert the same effects as BPA after all.
In conclusion, the long-term health risks posed by the current levels of exposure to BPA remain largely unknown. Although some epidemiological studies have shown correlations between BPA exposure and several medical conditions, research in humans is inconclusive. Yet, animal research shows reasons for concern as it indicates that low BPA levels within the range of human exposure can impact hormone balance, reproduction, neurodevelopment, and cancer risk.
The potential effects of BPA on human health must also be considered in the context of a widespread co-exposure to other environmental factors, which might worsen or mitigate these effects and make it difficult to draw definite conclusions. But given the potential of BPA to cause adverse effects, particularly during development, many scientists advocate for tighter regulations limiting exposure to BPA, especially in food packaging. In the meantime, avoiding plastic containers is the best way to limit exposure to BPA.
UPDATE (24 April 2023):
This review was updated to include an assessment of BPA safety published on 19 April 2023 by the EFSA. Three paragraphs describing the newly-assessed evidence and the conclusions of the EFSA’s re-evaluation were added at the end of the section discussing the disagreement between academics and regulators.
REFERENCES
- 1 – Krishnan et al. (1993) Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology.
- 2 – Lim et al. (2009) Potential Risk of Bisphenol a Migration From Polycarbonate Containers After Heating, Boiling, and Microwaving. Journal of Toxicology and Environmental Health.
- 3 – Hartle et al. (2016) The consumption of canned food and beverages and urinary Bisphenol A concentrations in NHANES 2003–2008. Environmental Research.
- 4 –Lehmler et al. (2018) Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013-2014. ACS Omega
- 5 – Colorado-Yohar et al. (2018) Concentrations of bisphenol-A in adults from the general population: A systematic review and meta-analysis. Science of the Total Environment.
- 6 – Vandenberg et al. (2010) Urinary, Circulating, and Tissue Biomonitoring Studies Indicate Widespread Exposure to Bisphenol A. Environmental Health Perspectives.
- 7 – Lee et al. (2918) Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother–neonate pairs. Science of the Total Environment.
- 8 – Plante et al. (2022) Killing two birds with one stone: Pregnancy is a sensitive window for endocrine effects on both the mother and the fetus. Environmental Research.
- 9 – Delbes et al. (2022) Effects of endocrine disrupting chemicals on gonad development: Mechanistic insights from fish and mammals. Environmental Research.
- 10 – Peretz et al. (2014) Bisphenol A and Reproductive Health: Update of Experimental and Human Evidence, 2007–2013. Environmental Health Perspectives.
- 11 – Crain et al. (2008) Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertility and Sterility.
- 12 – Cabaton et al. (2011) Perinatal Exposure to Environmentally Relevant Levels of Bisphenol A Decreases Fertility and Fecundity in CD-1 Mice. Environmental Health Perspectives.
- 13 – Patel et al. (2017) Bisphenol A Exposure, Ovarian Follicle Numbers, and Female Sex Steroid Hormone Levels: Results From a CLARITY-BPA Study. Endocrinology.
- 14 – Neff et al. (2019) Chronic Exposure of Mice to Bisphenol-A Alters Uterine Fibroblast Growth Factor Signaling and Leads to Aberrant Epithelial Proliferation. Endocrinology.
- 15 – Hunt et al. (2003) Bisphenol A Exposure Causes Meiotic Aneuploidy in the Female Mouse. Current Biology.
- 16 – Fernandez et al. (2010) Neonatal Exposure to Bisphenol A and Reproductive and Endocrine Alterations Resembling the Polycystic Ovarian Syndrome in Adult Rats. Environmental Health Perspectives.
- 17 – Newbold et al. (2009) Prenatal Exposure to Bisphenol A at Environmentally Relevant Doses Adversely Affects the Murine Female Reproductive Tract Later in Life. Environmental Health Perspectives.
- 18 – Vrooman et al. (2015) Estrogenic Exposure Alters the Spermatogonial Stem Cells in the Developing Testis, Permanently Reducing Crossover Levels in the Adult. PLOS Genetics.
- 19 – Tailor et al. (2020) Interactive Effects of Perinatal BPA or DES and Adult Testosterone and Estradiol Exposure on Adult Urethral Obstruction and Bladder, Kidney, and Prostate Pathology in Male Mice. International Journal of Molecular Sciences.
- 20 – Nicholson et al. (2018) Endocrine disruptor bisphenol A is implicated in urinary voiding dysfunction in male mice. American Journal of Physiology.
- 21 – Prins et al. (2018) Evaluation of Bisphenol A (BPA) Exposures on Prostate Stem Cell Homeostasis and Prostate Cancer Risk in the NCTR-Sprague-Dawley Rat: An NIEHS/FDA CLARITY-BPA Consortium Study. Environmental Health Perspectives.
- 22 – Vandenberg et al. (2007) Exposure to Environmentally Relevant Doses of the Xenoestrogen Bisphenol-A Alters Development of the Fetal Mouse Mammary Gland. Endocrinology.
- 23 – Durando et al. (2007) Prenatal Bisphenol A Exposure Induces Preneoplastic Lesions in the Mammary Gland in Wistar Rats. Environmental Health Perspectives.
- 24 – Acevedo et al. (2013) Perinatally Administered Bisphenol A as a Potential Mammary Gland Carcinogen in Rats. Environmental Health Perspectives.
- 25 – Tremblay-Franc et al. (2015) Dynamic Metabolic Disruption in Rats Perinatally Exposed to Low Doses of Bisphenol-A. Plos ONE.
- 26 – Alonso-Magdalena et al. (2010) Bisphenol A Exposure during Pregnancy Disrupts Glucose Homeostasis in Mothers and Adult Male Offspring. Environmental Health Perspectives.
- 27 – Zhang et al. (2022) Bisphenol a exposure decreases learning ability through the suppression of mitochondrial oxidative phosphorylation in the hippocampus of male mice. Food and Chemical Toxicology.
- 28 – Tavakkoli et al. (2020) Alteration of protein profile in cerebral cortex of rats exposed to bisphenol a: a proteomics study. Neurotoxicology.
- 29 – Martínez-Ibarra et al. (2021) Multisystemic alterations in humans induced by bisphenol A and phthalates: Experimental, epidemiological and clinical studies reveal the need to change health policies. Environmental Pollution.
- 30 – Shen et al. (2015) Higher Urinary Bisphenol A Concentration Is Associated with Unexplained Recurrent Miscarriage Risk: Evidence from a Case-Control Study in Eastern China. Plos ONE.
- 31 – Huo et al. (2015) Maternal urinary bisphenol A levels and infant low birth weight: A nested case–control study of the Health Baby Cohort in China. Environment International.
- 32 – Braun et al. (2011) Impact of Early-Life Bisphenol A Exposure on Behavior and Executive Function in Children. Pediatrics.
- 33 – Harley et al. (2013) Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environmental Research.
- 34 – Bhandari et al. (2013) Urinary Bisphenol A and Obesity in US Children. American Journal of Epidemiology.
- 35 – Trasande et al. (2012) Association Between Urinary Bisphenol A Concentration and Obesity Prevalence in Children and Adolescents. JAMA.
- 36 – Wang et al. (2012) Urinary Bisphenol A (BPA) Concentration Associates with Obesity and Insulin Resistance. The Journal of Clinical Endocrinology and Metabolism.
- 37 – Shankar & Teppala (2011) Relationship between Urinary Bisphenol A Levels and Diabetes Mellitus. The Journal of Clinical Endocrinology and Metabolism.
- 38 – Silver et al. (2011) Urinary Bisphenol A and Type-2 Diabetes in U.S. Adults: Data from NHANES 2003-2008. Plos ONE.
- 39 – Sun et al. (2014) Association of Urinary Concentrations of Bisphenol A and Phthalate Metabolites with Risk of Type 2 Diabetes: A Prospective Investigation in the Nurses’ Health Study (NHS) and NHSII Cohorts. Environmental Health Perspectives.
- 40 – Sabanayagam et al. (2013) Relationship between urinary bisphenol A levels and prediabetes among subjects free of diabetes. Acta Diabetologica.
- 41 – Bae et al. (2012) Associations of Bisphenol A Exposure With Heart Rate Variability and Blood Pressure. Hypertension.
- 42 – Bae & Hong (2015) Exposure to Bisphenol A From Drinking Canned Beverages Increases Blood Pressure. Hypertension.
- 43 – Melzer et al. (2010) Association of Urinary Bisphenol A Concentration with Heart Disease: Evidence from NHANES 2003/06. Plos ONE.
- 44 – Melzer et al. (2012) Urinary Bisphenol A Concentration and Risk of Future Coronary Artery Disease in Apparently Healthy Men and Women. Circulation.
- 45 – Ziv-Gal et al. (2016) Evidence for bisphenol A-induced female infertility: a review (2007–2016). Fertility and Sterility.
- 46 – Mok-Lin et al. (2010) Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. International Journal of Andrology.
- 47 – Bloom et al. (2011) Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertility and Sterility.
- 48 – Fujimoto et al. (2010) Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertility and Sterility.
- 49 – Ehrlich et al. (2012) Urinary Bisphenol A Concentrations and Implantation Failure among Women Undergoing in Vitro Fertilization. Environmental Health Perspectives.
- 50 – Galloway et al. (2010) Daily Bisphenol: An Excretion and Associations with Sex Hormone Concentrations: Results from the InCHIANTI Adult Population Study. Environmental Health Perspectives.
- 51 – Li et al. (2010) Urine bisphenol-A (BPA) level in relation to semen quality. Fertility and Sterility.
- 52 – Kortenkamp et al. (2022) Combined exposures to bisphenols, polychlorinated dioxins, paracetamol, and phthalates as drivers of deteriorating semen quality. Environment International.
- 53 – Sonavan et al. (2019) Bisphenol A co-exposure effects: a key factor in understanding BPA’s complex mechanism and health outcomes. Critical Reviews in Toxicology.
- 54 – Deepika et al. (2022) Unravelling sex-specific BPA toxicokinetics in children using a pediatric PBPK model. Environmental Research.
- 55 – Dere et al. (2018) Effects of continuous bisphenol A exposure from early gestation on 90 day old rat testes function and sperm molecular profiles: A CLARITY-BPA consortium study. Toxicology and Applied Pharmacology.
- 56 – Harley et al. (2013) Prenatal and Postnatal Bisphenol A Exposure and Body Mass Index in Childhood in the CHAMACOS Cohort. Environmental Health Perspectives.
- 57 – Lee et al. (2014) Prenatal bisphenol A and birth outcomes: MOCEH (Mothers and Children’s Environmental Health) study. International Journal of Hygiene and Environmental Health.
- 58 – Mendiola et al. (2010) Are Environmental Levels of Bisphenol A Associated with Reproductive Function in Fertile Men? Environmental Health Perspectives.
- 59 – Pan et al. (2019) Prenatal Bisphenol A exposure and early childhood neurodevelopment in Shandong, China. International Journal of Hygiene and Environmental Health.
- 60 – Gore et al. (2015) EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocrine Reviews.
- 61 – Welshons et al. (2006) Large Effects from Small Exposures. III. Endocrine Mechanisms Mediating Effects of Bisphenol A at Levels of Human Exposure. Endocrinology.
- 62 – Myers et al. (2009) Why Public Health Agencies Cannot Depend on Good Laboratory Practices as a Criterion for Selecting Data: The Case of Bisphenol A. Environmental Health Perspectives.
- 63 – Heindel et al. (2020) Data integration, analysis, and interpretation of eight academic CLARITY-BPA studies. Reproductive Toxicology.
- 64 – Manzoor et al. (2022) An insight into bisphenol A, food exposure and its adverse effects on health: A review. Frontiers in Nutrition.
- 65 – Corbett et al. (2022) Nutritional interventions to ameliorate the effect of endocrine disruptors on human reproductive health: A semi-structured review from FIGO. International Journal of Gynecology and Obstetrics.