- Health
Viral videos on Facebook promote unproven cancer cures; no evidence that pharmaceutical companies and the U.S. FDA are hiding the cure for cancer
Key takeaway
Medical advances, such as targeted therapies and immunotherapy, have improved disease prognosis and survival for cancer patients. Unproven remedies often touted as capable of curing any type of cancer, such as the ketogenic diet, ozone therapy, high-dose vitamin C, and adaptogens, don't work and can even be detrimental to health. Clinical trials for antineoplastons were halted due to serious problems that endangered the lives of patients.
Reviewed content
Verdict:
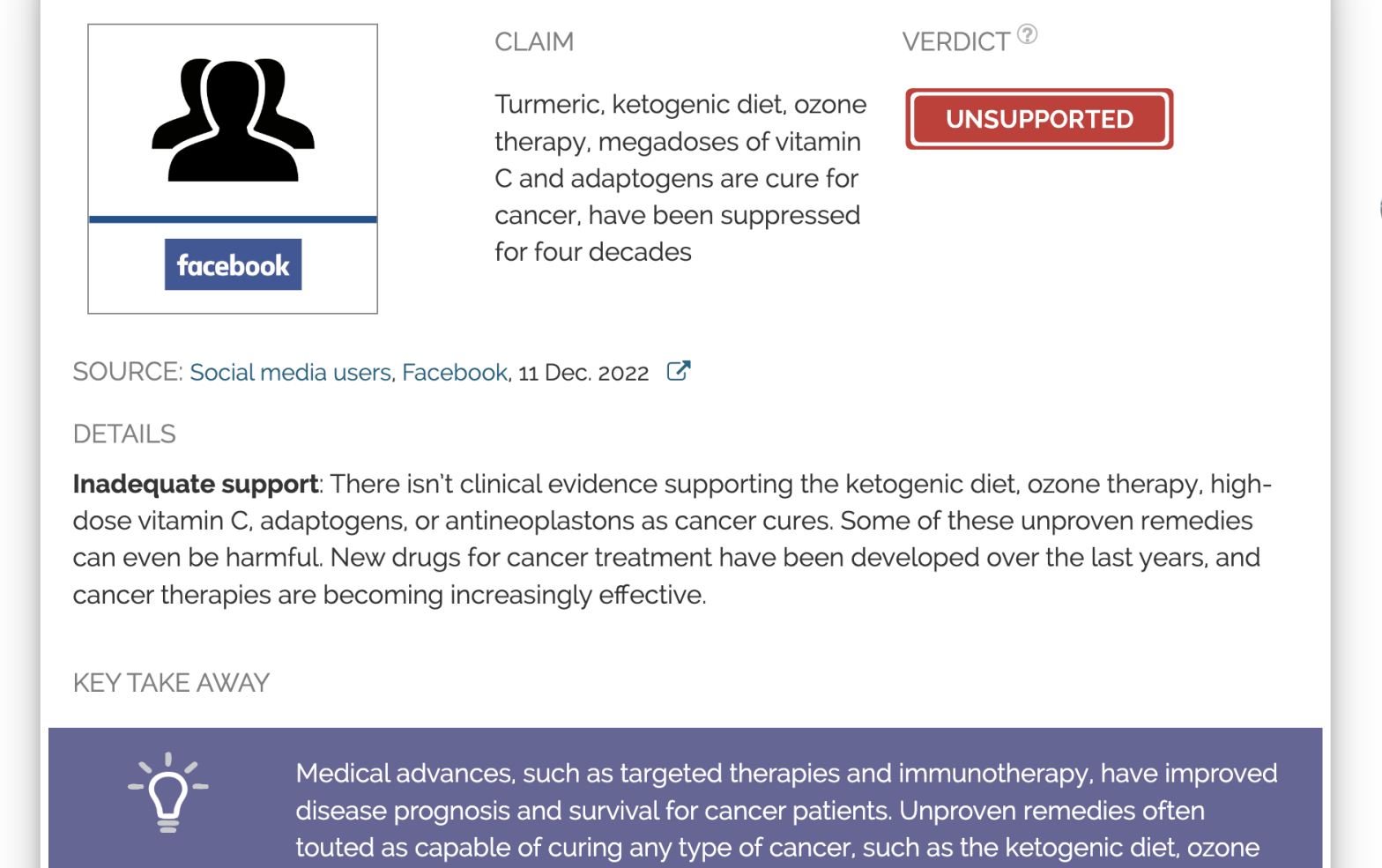
Claim:
Turmeric, ketogenic diet, ozone therapy, megadoses of vitamin C and adaptogens are cures for cancer that have been suppressed for four decades
Verdict detail
Inadequate support: There isn’t clinical evidence supporting the ketogenic diet, ozone therapy, high-dose vitamin C, adaptogens, or antineoplastons as cancer cures. Some of these unproven remedies can even be harmful. New drugs for cancer treatment have been developed over the last years, and cancer therapies are becoming increasingly effective.
Full Claim
Inexpensive treatments for cancer like turmeric have the same effect as anti-cancer drugs; ketogenic diet, ozone therapy, megadoses of vitamin C and adaptogens can cure cancer; the cure for cancer has been suppressed for four decades.
Review
Cancer is one of the leading causes of mortality worldwide, with 19.3 million new cases and almost 10 million deaths in 2020[1]. Strong public interest in cancer treatments has led to recurrent promotion of scientifically unproven cancer remedies on social media. Health Feedback previously debunked several of them, including soursop, vitamin C, cannabis, ozone therapy, turmeric, and orgone.
Unproven cancer remedies, whether they are a genuine attempt to do good or outright quackery, have an overall negative impact on patients. A 2018 study published in the journal JAMA found that patients who choose to use unproven remedies for cancer treatment are more likely to reject conventional therapies. They are also more than twice as likely to die over the same period compared to those using conventional treatments[2]. And less hopeful prognosis of cancer has been associated with greater likelihood to use unproven remedies[2,3].
One tactic that is associated with promoting unproven cancer cures is the dismissal of conventional cancer therapies, like chemotherapy or radiotherapy, as dangerous or harmful.
Another tactic is to cite the high cost of cancer treatments and suggest that an unproven cancer cure offers the same effect as conventional treatments without carrying the same financial cost. For example, this video claimed that turmeric has a similar effect to Nexavar, a drug used to treat several types of cancer, while being 1,000 times cheaper. However, no clinical evidence so far supports the use of turmeric as a cancer therapy, as explained in this claim review by Health Feedback.
In this article we will discuss the lack of evidence for unproven cancer remedies that were promoted in several videos that went viral on social media platforms in late 2022.
Use of ketogenic diet, intermittent fasting or ozone for cancer treatment isn’t supported by clinical evidence
A viral video in Spanish, posted on Facebook in December 2022, made a number of false claims about cancer. The first is that consuming sugar favors the development of cancer because of the Warburg effect. This claim is based on the observation that cancer cells ferment sugar at a higher rate than healthy cells[4].
Under normal circumstances, healthy human cells tend to obtain energy by consuming nutrients through aerobic respiration, which occurs in the presence of oxygen. If oxygen is in short supply, cells may resort to fermentation instead. Aerobic respiration is more efficient than fermentation because it produces more energy, so healthy cells preferentially obtain energy by aerobic respiration.
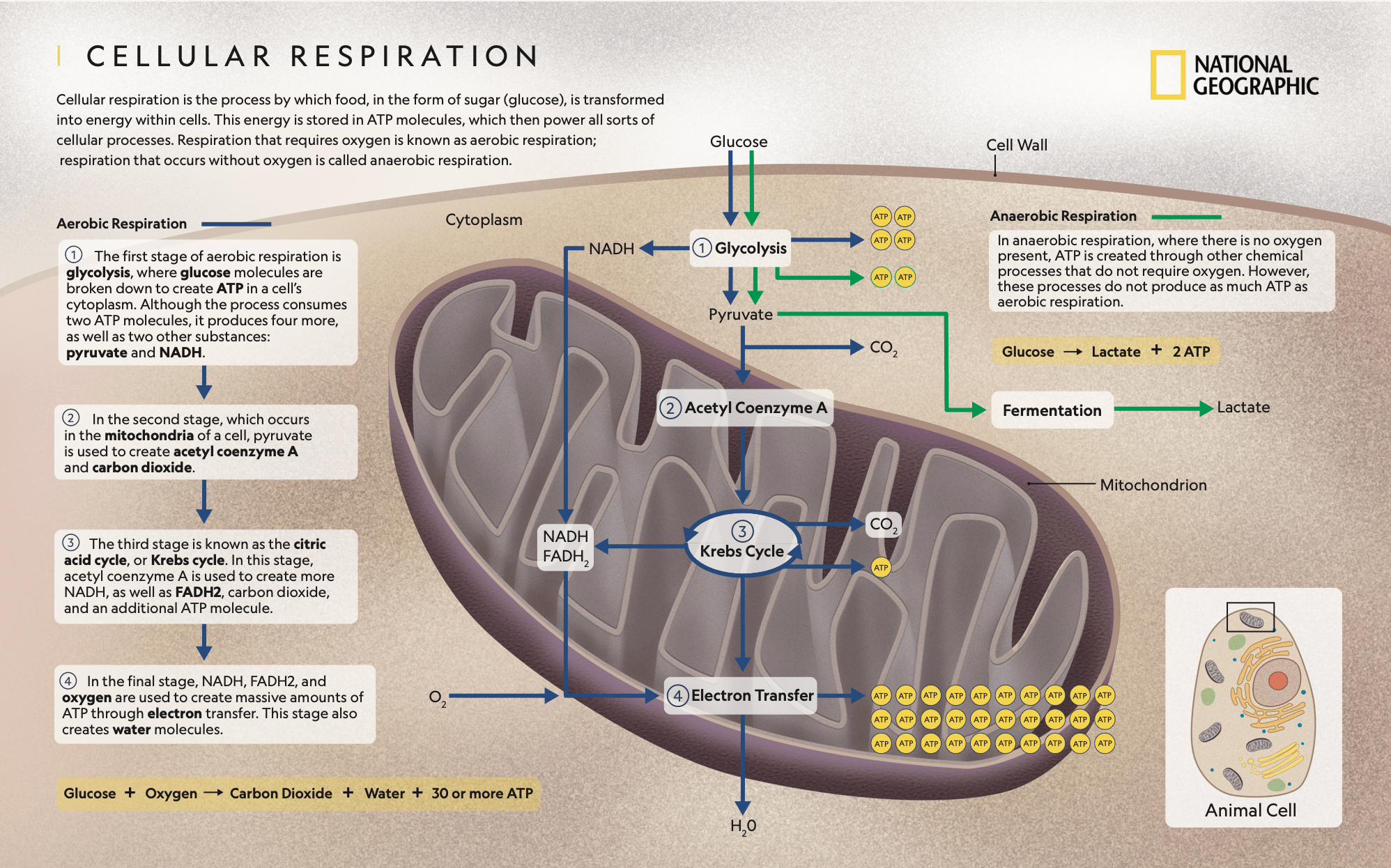
Figure 1. Diagram showing the processes involved in cellular respiration. By National Geographic.
A common instance of healthy cells performing fermentation is that of skeletal muscle cells. When a person performs intense exercise, skeletal muscle cells require a high amount of energy, so they consume more oxygen than the body can take in. When oxygen levels drop, skeletal muscle cells switch to fermentation to obtain energy. This produces lactic acid within the cells, which causes stiffness and cramps after intense exercise.
German biochemist Otto Warburg discovered in the 1920s that cancer cells tend to obtain energy by fermenting glucose, even when the levels of oxygen favor aerobic respiration over fermentation[4]. Based on this finding, Warburg hypothesized that changes in cell metabolism were the cause of cancer.
We now know that cancer is caused by genetic mutations that alter how cells regulate their development, leading to uncontrolled cell growth, which can also alter cell metabolism. The increase of glucose fermentation in cancer cells is known as the Warburg effect[5].
The video claimed that a ketogenic diet and intermittent fasting would be useful as a cancer treatment. Ketogenic diet is based on restricting the intake of sugars in favor of proteins and fats. A literature review regarding the effect of the ketogenic diet on cancer development found a lack of randomized controlled trials. In other words, there is a lack of reliable clinical evidence in humans to recommend the ketogenic diet as a treatment against cancer[6].
Intermittent fasting consists of cyclic periods of caloric restriction, such as 24 hours of fasting followed by 24 hours of normal diet. A literature review regarding the effectiveness of intermittent fasting as a cancer treatment found that the effects on human cancer incidence and development remain unknown due to a lack of high-quality randomized clinical trials[7], similar to the ketogenic diet.
The video also cited ozone therapy and oxygen as cancer treatments. These recommendations are based on the previously mentioned ability of cancer cells to ferment glucose in the presence of oxygen, which has led to the false belief that oxygen kills cancer cells. There is no scientific evidence for this claim, as explained in this claim review by Health Feedback. There isn’t clinical evidence to support the usefulness of oxygen therapy or ozone therapy as a cancer treatment. After six decades of research, even preclinical studies showing positive results are scarce[8].
Also, according to the FDA, “Ozone is a toxic gas with no known useful medical application in specific, adjunctive, or preventive therapy”. The FDA also warns that the concentrations at which ozone has germicidal properties are “far greater than that which can be safely tolerated by man and animals”.
Little clinical evidence supports the use of high-dose intravenous vitamin C to treat cancer
Another of the unproven cancer remedies mentioned in the video is “megadoses of vitamin C”, referring to high-dose intravenous vitamin C. The alleged therapeutic benefits of vitamin C were promoted by chemist Linus Pauling, who performed several studies on its use in cancer treatment during the 1970s[9,10]. However, the experimental design of both Pauling’s studies contained numerous flaws, as explained in this article for Science-Based Medicine by oncologist David Gorski:
“The study’s flaws, which were too numerous to mention, rendered its results essentially meaningless. If you want a quote from his original paper that shows this better than anything, here it is: ‘We believe that the ascorbate[vitamin C]-treated patients represent a random selection of all the terminal patients in the hospital, even though no formal randomization process was used.’ Suffice it to say that, in a clinical trial, it is not sufficient to ‘believe’ that your groups were properly randomized and matched. You have to show it.
[…] Undeterred, they published a follow-up study in 1978 that purported to confirm the findings of their 1976 study. It was little better. Pauling and Cameron used many of the same patients and selected a different control group, but the patients were still not matched for stage of cancer, age, or performance or nutritional status. It, too, was a shockingly poorly-designed study, even for a retrospective study.”
Two randomized, controlled clinical trials conducted at the Mayo Clinic, published in 1979 and 1985, failed to replicate the benefits of vitamin C on cancer patients reported by Pauling’s earlier studies[11,12]. There is no reliable clinical evidence to support the intravenous administration of vitamin C as a therapy for cancer, as explained in this claim review by Health Feedback.
In addition, high doses of vitamin C can be harmful. The video recommended doses of 50 grams per week for several months, which is well above the recommended daily doses of 0.075 grams per day for women and 0.09 grams per day for men.
No clinical evidence that plant extracts known as adaptogens are effective at treating cancer
Last but not least, the video also recommended the consumption of adaptogens for treating cancer. Adaptogens are plant extracts that allegedly improve the body’s response to stress and restore physiological functioning to its normal level.
According to a 2018 review on the pharmacological uses of adaptogens, only a few preclinical studies have tested the effect of these herbal remedies on cancer cells[13]. These studies were performed in test tubes or mice, and therefore don’t show the effect of adaptogens on the human body and can’t be used to confirm any potential health benefit in people.
The video specifically mentioned three edible mushrooms as alleged cancer remedies: shiitake (Lentinula edodes), maitake (Grifola frondosa), and reishi (Ganoderma lucidum). However, no clinical studies support their use as a cancer therapy.
Shiitake was evaluated in a clinical trial with only seven participants that tested the mushroom’s effect when administered together with chemotherapy[14]. The study found that consumption of shiitake was safe and improved the quality of life and activity of the immune system of patients undergoing chemotherapy. However, the study’s authors cautioned that there was a need for more clinical studies with larger numbers of participants.
A Phase 1/2 clinical trial tested the effect of maitake extract in breast cancer patients. The study was small, with only 34 participants, and not randomized. The authors reported a mix of stimulatory and suppressive effects on the immune system, which could in turn influence different immune cells’ ability to fight cancer. The authors cautioned that “botanical agents produce more complex effects than assumed, and may depress as well as enhance immune function”[15].
Finally, a Cochrane Systematic Review which evaluated five randomized clinical trials in cancer patients administered reishi found insufficient evidence to recommend the use of this herbal remedy, noting that the published studies were of poor methodological quality[16].
In conclusion, the evidence on the effectiveness of shiitake, maitake and reishi in the treatment of cancer is limited to small clinical studies, which tested their effect on the immune system, but didn’t report an anti-cancer effect of these plant extracts.
The FDA isn’t suppressing antineoplastons as cancer therapy; clinical trials were halted after serious deficiencies led to the death of a child
Antineoplastons are another unproven treatment for cancer that some viral videos (like this one and this one) have claimed the FDA has suppressed for over four decades. Antineoplastons are chemical compounds found in urine and blood, consisting of amino acids (organic molecules that are the building blocks of proteins) and peptides (compounds consisting of two or more amino acids).
The use of antineoplastons for the treatment of cancer was first proposed in the 1970s by the physician Stanisław Burzyński. While studying the composition of the urine of cancer patients, Burzyński observed certain substances made of amino acids, which he named “antineoplastons”. Burzyński hypothesized that antineoplastons played a role in the immune system’s response to cancer, but that the human body was unable to produce them in sufficient quantities. He began human trials, first with antineoplastons extracted from urine, then by synthesizing them in the laboratory.
However, after several decades of research, there is no evidence that antineoplastons play a role in the immune system’s response to cancer. Moreover, according to the non-profit organization Cancer Research U.K., most of the positive reported results from antineoplaston trials come from Burzyński’s own clinic. These positive results came from Phase 1 and Phase 2 clinical trials, which weren’t randomized or controlled, as well as from case reports on individual patients.
According to the U.S. National Cancer Institute, no randomized, controlled trials of antineoplastons have been published in peer-reviewed scientific journals. Furthermore, treatment with antineoplastons causes mild to severe side effects, including anemia, abnormally high levels of calcium in the blood, and high blood pressure, among others.
Burzyński and his clinic were prosecuted several times in relation to the unlawful use and advertising of antineoplastons as an unapproved cancer therapy. Several warnings were issued by the FDA and the Texas Medical Board related to the advertisement of investigational drugs as being “safe and effective”.
The claim that the FDA is hiding antineoplastons from the general public as a cure for cancer is plainly false, since several clinical trials with antineoplastons were authorized. For example, Phase 2 clinical trials using antineoplastons to treat multiple myeloma, brain tumors, or lung cancer were registered. All these trials were authorized to be performed at the Burzynski Clinic in Houston, Texas. Furthermore, the FDA agreed to antineoplaston treatment for several cancer patients through “compassionate use exemptions”, which undermines the claim that the FDA is hiding or suppressing the use of this treatment.
Clinical trials with antineoplastons were paused in 2013 following the death of a six-year-old child during the trials. A subsequent inspection by the FDA found serious problems in how the trials were conducted. These included destroying patient medical records; altering trial results to make them appear more favorable; failing to report life-threatening side effects during trials; and overdosing patients with drugs, as reported by USA Today.
At least 18 patients undergoing antineoplaston treatment experienced hypernatremia (excess sodium in the blood), as stated in this FDA report. In fact, the death of the six-year-old child that led to the FDA inspection was caused by hypernatremia. Sodium levels in these patients were so high because antineoplastons are mixed with sodium salts, as indicated in patents on antineoplastons like this one. Side effects due to the high sodium content of antineoplaston formulations have been known for many years, as shown in this article published in The Cancer Letter in 1998.
Overall, the only evidence of the alleged benefits of antineoplastons in cancer treatment comes from seriously flawed clinical trials that endangered the lives of their participants, run by the same person who made the claim.
The evidence we have contradicts the claim that the FDA is suppressing this alleged cure for cancer. Many clinical trials were authorized over the years until the death of a participant exposed serious deficiencies, and the records of these clinical trials are publicly available on the Internet.
No evidence that pharmaceutical companies are hiding the cure for cancer; new drugs and therapies have improved disease prognosis
A persistent myth about cancer treatments is that pharmaceutical companies benefit from hiding the “cure for cancer” and promoting chemotherapy because it is more lucrative. This claim was widely promoted in videos shared on social media platforms (like this one or this one).
Cancer is the uncontrollable growth of cells in any of the body’s tissues and the spread of these cells to other parts of the body. Unfortunately, there isn’t a “one true cure” for cancer, as different types of cancer require different forms of treatment. Therefore, to speak of a “cure for cancer” as a silver bullet that can treat any cancer is misleading (see an example here).
On closer scrutiny, the logic of the claim that pharmaceutical companies hide the cure for cancer for financial reasons doesn’t hold up. For starters, it is extremely unlikely that such a secret could be kept for a long time, given that the number of people who work for pharmaceutical companies runs in the hundreds of thousands[17].
Also, the researchers working for pharmaceutical companies may have reasons other than money to find a cure for cancer. Everyone knows someone affected by the disease—it makes no sense for researchers to risk the lives of their loved ones by hiding successful treatments. On the other hand, as the U.K-based charity Worldwide Cancer Research pointed out, pharmaceutical companies stand to profit much more from a cancer cure rather than hide it.
Recent advances in cancer research have enabled the development of new therapies, improving the disease prognosis and patient survival. Here is a list of the main hallmarks in cancer treatment achieved during the last decades, many of which have been made possible by advances in genetics.
One of the recent developments in cancer treatment are targeted therapies. This treatment takes advantage of information about specific genes and proteins altered in a person’s cancer cells. This allows the drugs to kill the cancer cells while minimizing damage to healthy cells and tissues. The FDA has approved many drugs based on targeted therapies, some of which are useful for treating more than one type of cancer. The first of these drugs approved by the FDA was trastuzumab, first approved in 1998 for treating breast cancer, and subsequently approved for treating metastatic stomach cancer.
Another recent development in cancer treatment is immunotherapy, which enhances the immune system’s natural ability to detect and destroy cancer cells. This therapy overcomes alterations in cancer cells that allow them to evade the immune system or make them resistant to anti-cancer drugs[18]. This page shows a list of immunotherapies that the FDA has approved since 2014.
Vaccines have also been developed to prevent certain types of cancer. One example is Gardasil, which prevents infection by several strains of human papillomavirus (HPV). This virus is responsible for most cases of invasive cervical cancer worldwide[19]. According to the U.S. Centers for Disease Control and Prevention, 10 years after Gardasil was recommended in the U.S., infections of the strains targeted by the vaccine declined by 86% in teen girls aged 14 to 19 years and by 71% in women in their early 20s.
Drugs and therapies have been developed over the last decades which enable more effective cancer treatment, as well as vaccines that prevent new cases of cancer, which shows that pharmaceutical companies are actively researching cures for the disease.
Conclusion
In conclusion, there is a lack of clinical evidence supporting the use of ketogenic diet, ozone therapy, high-dose vitamin C and adaptogens as effective cancer treatments. Clinical trials of antineoplastons were discontinued due to serious problems that endangered the lives of patients. These alleged cancer cures, often touted as being able to cure any type of cancer, don’t work and can be harmful.
UPDATE (27 December 2022):
This review was updated to improve clarity and to provide an additional reference regarding the high sodium content in antineoplastons from the publication The Cancer Letter. We also corrected our report of the Phase 1/2 clinical trial on maitake extract. The review originally stated that “A stimulatory effect on the immune system was observed”. This is incorrect; the authors reported a mix of stimulatory and suppressive effects during the study.
REFERENCES
- 1 – Sung et al. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians.
- 2 – Johnson et al. (2018) Complementary Medicine, Refusal of Conventional Cancer Therapy, and Survival Among Patients With Curable Cancers. JAMA Oncology.
- 3 – Risberg et al. (1997) Why are cancer patients using non-proven complementary therapies? A cross-sectional multicentre study in Norway. European Journal of Cancer.
- 4 – Hay (2016) Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy?. Nature Reviews Cancer.
- 5 – Liberti and Locasale (2016) The Warburg Effect: How Does it Benefit Cancer Cells?. Trends in Biochemical Sciences.
- 6 – Weber et al. (2020) Ketogenic diet in the treatment of cancer – Where do we stand?. Molecular Metabolism.
- 7 – Clifton et al. (2021) Intermittent fasting in the prevention and treatment of cancer. CA: A Cancer Journal for Clinicians.
- 8 – Noci and Pinto-Bonilla (2021) Systemic Review: Ozone: A Potential New Chemotherapy. International Journal of Molecular Sciences.
- 9 – Cameron et al. (1975) The orthomolecular treatment of cancer: III. Reticulum cell sarcoma: Double complete regression induced by high-dose ascorbic acid therapy. Chemico-Biological Interactions.
- 10 – Cameron and Pauling (1976) Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proceedings of the National Academy of Sciences.
- 11 – Creagan et al. (1979) Failure of High-Dose Vitamin C (Ascorbic Acid) Therapy to Benefit Patients with Advanced Cancer — A Controlled Trial. The New England Journal of Medicine.
- 12 – Moertel et al. (1985) High-Dose Vitamin C versus Placebo in the Treatment of Patients with Advanced Cancer Who Have Had No Prior Chemotherapy — A Randomized Double-Blind Comparison. The New England Journal of Medicine.
- 13 – Liao et al. (2018) A preliminary review of studies on adaptogens: comparison of their bioactivity in TCM with that of ginseng-like herbs used worldwide. Chinese Medicine.
- 14 – Yamaguchi et al. (2011) Efficacy and safety of orally administered Lentinula edodes mycelia extract for patients undergoing cancer chemotherapy: a pilot study. The American Journal of Chinese Medicine.
- 15 – Deng et al. (2009) A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: immunological effects. Journal of Cancer Research and Clinical Oncology.
- 16 – Jin et al. (2016) Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database of Systematic Reviews.
- 17 – Grimes (2016). On the Viability of Conspiratorial Beliefs. PLOS ONE.
- 18 – Tan et al. (2020) Cancer immunotherapy: Pros, cons and beyond. Biomedicine & Pharmacotherapy.
- 19 – Chan et al. (2019) Human Papillomavirus Infection and Cervical Cancer: Epidemiology, Screening, and Vaccination—Review of Current Perspectives. Journal of Clinical Oncology.