- Health
Contrary to claim in viral Facebook post, the flu vaccine does work and certain types of cancers can be cured
Key takeaway
The flu vaccine helps to reduce the incidence and severity of influenza, although its effectiveness varies between seasons depending on the selection of the flu vaccine virus strains. Cancer is a complex family of diseases for which multiple forms of treatment are available, although the success of such treatments can vary significantly, depending on factors like the type and stage of the cancer. There are a proportion of cancer patients who have achieved complete remission after treatment and remain cancer-free for the rest of their lives, who are thereby considered to be cured. And while there is much we do not know about COVID-19, our prior knowledge of how vaccines work is sufficient to allow us to develop a potential vaccine against the disease.
Reviewed content
Verdict:
Claim:
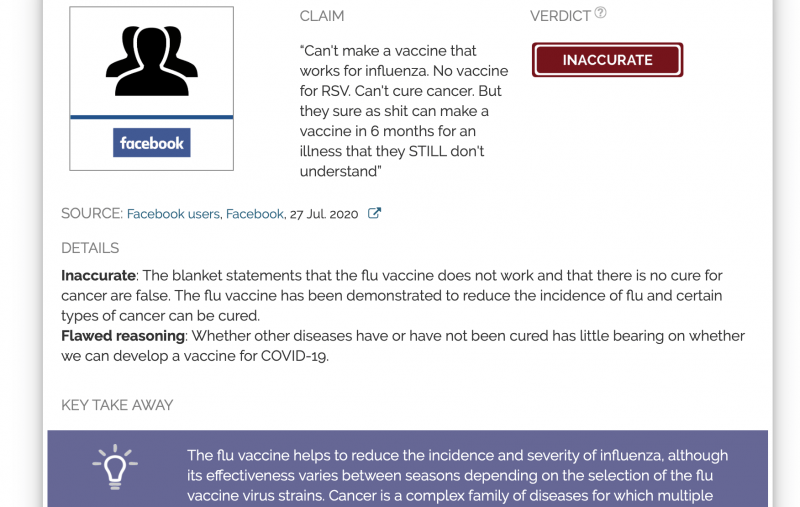
“Can't make a vaccine that works for influenza. No vaccine for RSV. Can't cure cancer. But they sure as shit can make a vaccine in 6 months for an illness that they STILL don't understand”
Verdict detail
Inaccurate: The blanket statements that the flu vaccine does not work and that there is no cure for cancer are false. The flu vaccine has been demonstrated to reduce the incidence of flu and certain types of cancer can be cured.
Flawed reasoning: Whether other diseases have or have not been cured has little bearing on whether we can develop a vaccine for COVID-19.
Full Claim
“Can't make a vaccine that works for influenza. No vaccine for RSV. Can't cure cancer. But they sure as shit can make a vaccine in 6 months for an illness that they STILL don't understand”
Review
A Facebook post published at the end of July 2020 expressed skepticism at how we could “make a vaccine in 6 months for an illness that [we] STILL don’t understand”—heavily implied to be COVID-19—by claiming that we “can’t make a vaccine that works for influenza” and “can’t cure cancer”. Posts resharing this message have been circulating in Facebook groups, including those which oppose vaccination and promote conspiracy theories, receiving more than 9,900 interactions on Facebook in just over a week according to CrowdTangle.
As this review explains below, the post relies on a mix of inaccurate information and flawed reasoning to question the possibility of developing a COVID-19 vaccine within six months. It is not clear based on what evidence the post asserts that scientists are sure to perform this feat in six months, which is an extremely short timeline; in fact, the general scientific consensus is that a vaccine would likely take at least a year to develop, if not more. The post also makes a reference to “drinking the Kool-Aid”, implying that scientists working to develop a COVID-19 vaccine are attempting to hoodwink and harm the general public, although the post does not explain what motive scientists would have for doing so.
The flu vaccine works to reduce the incidence and severity of flu, although it does not confer complete protection
The blanket statement that we have no vaccine that works for influenza is inaccurate. Studies have shown that the flu vaccine can reduce the incidence and severity of flu[1-6]. This was also the conclusion of a previous Health Feedback review regarding the efficacy of the flu shot in the elderly.
However, it is true that compared to other vaccines like the measles vaccine, which has about 93% effectiveness with one dose and 97% with two doses, the flu vaccine is not as effective. Furthermore, the effectiveness of the flu vaccine fluctuates from season to season. Based on data from the U.S Centers for Disease Control and Prevention (CDC), vaccine effectiveness over the past 10 flu seasons ranged from 19 to 60%.
Vaccine effectiveness is determined by the strains of inactivated flu viruses which are included in the shot, which may differ from season to season, depending on factors like genetic changes in the circulating flu viruses. The selection of flu vaccine strains is based on recommendations made by scientists using data from flu surveillance laboratories around the world, in order to understand which flu strains are circulating and causing illness. The recommendations are then issued by the WHO, although the health authority for each country makes the final decision regarding which flu strains are included in the vaccines licensed for their country. In the case of the U.S., this decision is made by the CDC.
There are instances where strain predictions for a particular flu season turned out to be a mismatch, which likely led the effectiveness of the flu vaccine to be lower than expected. However, even a mismatched flu vaccine can provide partial protection against the flu. The post’s implication that the vaccine does not work because it does not produce perfect results is an example of the nirvana fallacy, in which a realistic solution to a problem is dismissed simply because it does not achieve an impossible ideal.
No vaccine for the respiratory syncytial virus (RSV) exists yet
RSV is one of the viruses responsible for the common cold. It usually produces mild illness in older children and adults. But like the flu, certain parts of the population, such as very young children and the elderly, are at a higher risk of complications like pneumonia. According to the CDC:
“Over 57,000 hospitalizations, 500,000 emergency department visits and 1.5 million outpatient clinic visits among children <5 years of age are attributed to respiratory syncytial virus (RSV) infections each year in the United States. RSV-associated deaths among children <5 years of age are thought to be uncommon, estimated at 100-500 per year. Among US adults, an estimated 177,000 hospitalizations and 14,000 deaths associated with RSV infections occur annually. However, these are likely underestimates of RSV-associated deaths.”
It is true that no vaccine for RSV exists yet, although a synthetic antibody called palivizumab can be used to prevent RSV infection in high-risk infants (prophylaxis), such as those who were born prematurely or with heart conditions. However, its effects are short-lived and it cannot be used to treat an infection that has already begun. Patients with RSV infection receive mainly supportive care, as there is a lack of effective drugs for treating the infection. Overall, given the lack of effective therapies for the disease, developing a vaccine to prevent RSV infection, especially in at-risk populations, remains an important goal.
Some vaccine candidates against RSV are now in clinical trials[7]. Safety considerations are paramount—in 1966, young children who had received an inactivated RSV vaccine in the U.S. developed more severe illness upon infection with RSV compared to unvaccinated children. However, based on new developments in immunology and additional studies, scientists were able to uncover the mechanism behind the enhanced disease severity induced by the vaccine[8]. This knowledge will enable scientists to work towards designing safer RSV vaccine candidates[9].
Cancer is not a single disease, but a catch-all term for many different diseases; some are easier to treat than others
Broadly speaking, cancer is simply the uncontrolled growth and proliferation of cells as a result of gene mutations. However, there are many different mechanisms which underpin this overgrowth[10] which result from different combinations of genetic mutations that vary from person to person. This means that cancer is essentially a family of different diseases rather than a single disease, some of which are more aggressive and difficult to treat. While there is no universal cure for cancer—and we are very unlikely to find one, as a previous Health Feedback review explained—there are treatments available, including chemotherapy, radiotherapy, surgery, and immunotherapy, although anticancer therapy has varying degrees of success, depending on factors like the type of cancer and how advanced the disease is at the time of treatment.
For instance, acute lymphoblastic leukemia, a type of blood cancer affecting white blood cells called lymphocytes, is the most common childhood cancer, but about 90% of children are cured with treatment. On the other hand, glioblastoma multiforme, a type of brain cancer, is much more dangerous. According to the National Organization for Rare Diseases, “The average survival time for patients with glioblastoma who have undergone combination treatments of surgery, chemotherapy, and radiotherapy is 14.6 months.”
In short, the post is inaccurate, as it oversimplifies cancer by characterizing it as a single disease and it does not acknowledge that the outcome of treatment for different types of cancer can be highly variable. Finally, treatment can produce complete, lifelong remission in some patients depending on the type of cancer, and such patients are effectively considered to be “cured”.
We can develop a vaccine or treatment for a disease, even if we don’t understand the disease fully
COVID-19 remains a mystery to scientists, ranging from how the causal agent SARS-CoV-2 evolved to how it can be treated and prevented in humans. However, scientists have demonstrated that previous work in related coronaviruses[11], such as SARS-CoV-1[12] and MERS-CoV[13], can give us a headstart on developing a potential COVID-19 vaccine.
Contrary to the implied claim, we do not need to know everything or even a lot about a disease in order to treat or prevent it. The basic principle of vaccination is to expose a person to an agent, such as a protein from a particular pathogen, that can induce immunity against a pathogen, but does not itself cause the disease. In fact, society had been aware of this concept long before immunology became a field of study, as illustrated by the work of English physician Edward Jenner in the late 1700s, who successfully vaccinated a child against smallpox by inoculating him with cowpox material. Furthermore, at the time that Jenner performed his experiments in vaccination, germ theory—the accepted scientific theory that microorganisms can cause disease—had not been formulated and Jenner did not know that smallpox was caused by a virus. Germ theory was primarily formulated only in the late 1800s by scientists, among whom were Louis Pasteur and Robert Koch.
In summary, while much about COVID-19 and the virus SARS-CoV-2 still eludes us, we do have an understanding of how to induce immunity against a pathogen, thanks to the decades of research in microbiology and immunology, which can be applied to new pathogens. Hence the post places an unwarranted amount of necessity in understanding a disease fully in order to develop a vaccine or treatment. While there is no doubt that having more knowledge about a disease is helpful in developing treatment and prevention strategies, incomplete knowledge is not in itself an insurmountable obstacle.
REFERENCES
- 1 – Flannery et al. (2017) Influenza Vaccine Effectiveness Against Pediatric Deaths: 2010–2014. Pediatrics.
- 2 – Arriola et al. (2017) Influenza Vaccination Modifies Disease Severity Among Community-dwelling Adults Hospitalized With Influenza. Clinical Infectious Diseases.
- 3 – Thompson et al. (2018) Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine.
- 4 – Groenwold et al. (2009) Impact of influenza vaccination on mortality risk among the elderly. European Respiratory Journal.
- 5 – Foppa et al. (2015) Deaths averted by influenza vaccination in the U.S. during the seasons 2005/06 through 2013/14. Vaccine.
- 6 – Godoy et al. (2018) Influenza vaccine effectiveness in reducing severe outcomes over six influenza seasons, a case-case analysis, Spain, 2010/11 to 2015/16. Eurosurveillance.
- 7 – Mazur et al. (2018) The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. The Lancet Infectious Diseases.
- 8 – Delgado et al. (2008) Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nature.
- 9 – Acosta et al. (2016) Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease. Clinical and Vaccine Immunology.
- 10 – Hanahan and Weinberg. (2011) Hallmarks of Cancer: The Next Generation. Cell.
- 11 – Padron-Regalado E. (2020) Vaccines for SARS-CoV-2: Lessons from Other Coronavirus Strains. Infectious Diseases and Therapy.
- 12 – Subbarao K. (2020) SARS-CoV-2: A New Song Recalls an Old Melody. Cell Host and Microbe.
- 13 – Folegatti et al. (2020) Safety and immunogenicity of a candidate Middle East respiratory syndrome coronavirus viral-vectored vaccine: a dose-escalation, open-label, non-randomised, uncontrolled, phase 1 trial. The Lancet Infectious Diseases.