- Health
Animal studies included in Pfizer COVID-19 vaccine approval application showed no signs of reproductive toxicity
Key takeaway
Safety monitoring and clinical data continue to show that the mRNA COVID-19 vaccines are safe for pregnant women and protect them from pregnancy complications due to COVID-19. No evidence from animal or clinical studies suggests that these vaccines cause or increase fertility problems or negative pregnancy outcomes.
Reviewed content
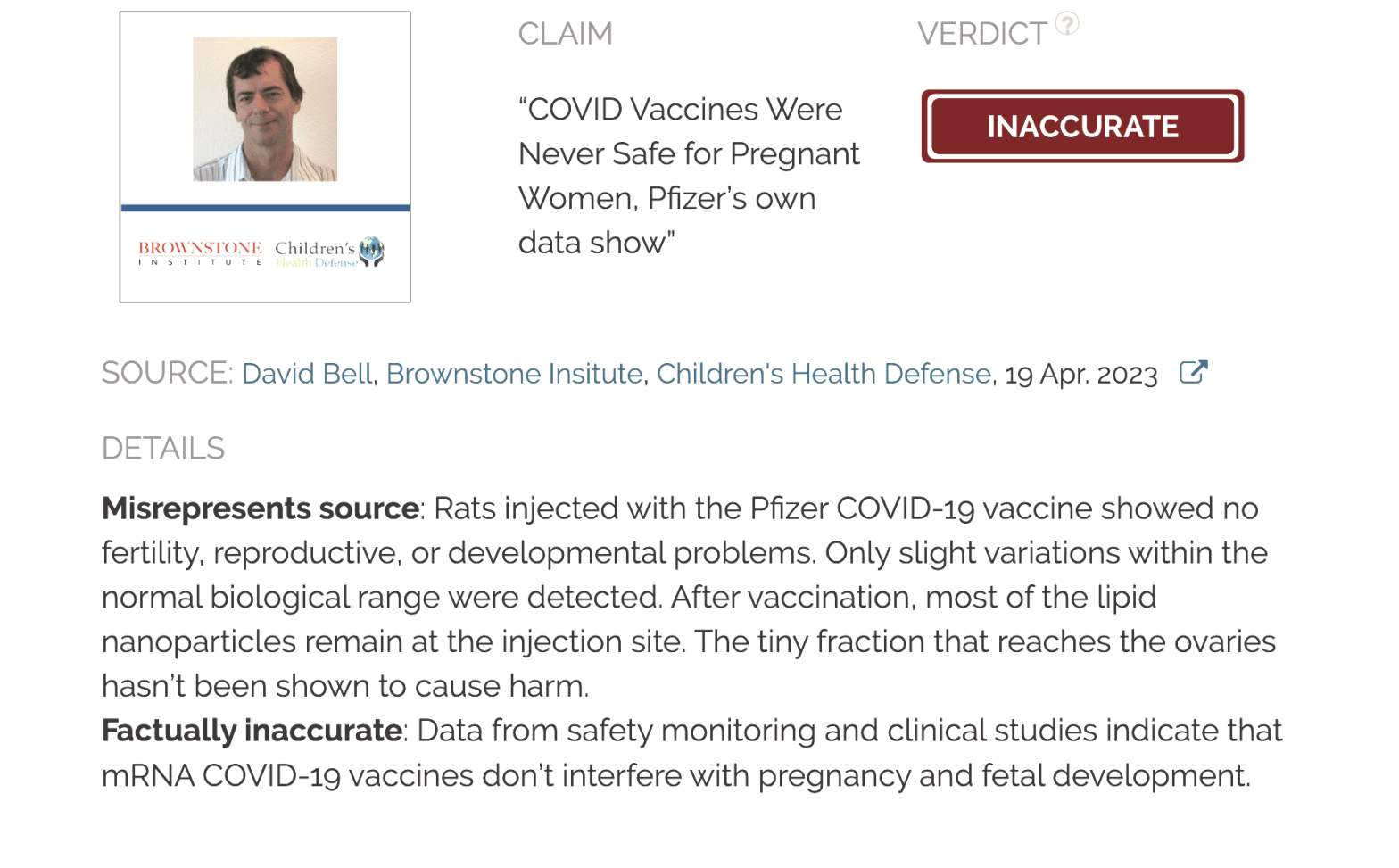
Verdict:
Claim:
“COVID Vaccines Were Never Safe for Pregnant Women, Pfizer’s own data show”
Verdict detail
Misrepresents source: Rats injected with the Pfizer COVID-19 vaccine showed no fertility, reproductive, or developmental problems. Only slight variations within the normal biological range were detected. After vaccination, most of the lipid nanoparticles remain at the injection site. The tiny fraction that reaches the ovaries hasn’t been shown to cause harm.
Factually inaccurate: Data from safety monitoring and clinical studies indicate that mRNA COVID-19 vaccines don’t interfere with pregnancy and fetal development.
Full Claim
“the pre-implantation loss rate and the number of abnormalities per fetus [...] were significantly higher for vaccinated rats than for unvaccinated rats”; “the vaccine will travel throughout the body after injection, and is found not only at the injection site, but in all organs tested, with high concentration in the ovaries, liver, adrenal glands, and spleen".
Review
On 19 April 2023, the Brownstone Institute published an article claiming that Australian regulatory authority the Therapeutic Goods Administration (TGA) had evidence that the Pfizer-BioNTech COVID-19 vaccine was unsafe during pregnancy before approving it provisionally on 27 August 2021.
Through the anti-vaccine group Children’s Health Defense, which republished the article here, the claim received around 68,000 views and more than 1,300 shares on Twitter.
The claim is based on a document (FOI 2389, document 6) containing nonclinical data on the COVID-19 vaccine safety and efficacy and submitted by Pfizer to the TGA on 8 January 2021 as part of the approval application. This document was confidential until the TGA released it on 15 July 2021 under a Freedom of Information request.
Specifically, the article focused on two experiments conducted in rats, one that evaluated the distribution of lipid nanoparticles through the body following injection and another that evaluated the effects of the vaccine on reproduction. Based on these data, the Brownstone article claimed that the COVID-19 vaccine accumulated in the ovaries and increased the rate of pregnancy loss and fetal abnormalities in rats. This, it claimed, provided evidence that COVID-19 vaccination in pregnant women “is high-risk and not justified”.
Pfizer’s approval applications for the COVID-19 vaccines have become a regular subject of misinformation for individuals who misuse and distort the data presented in them to support the narrative that the mRNA COVID-19 vaccine is unsafe. In the case of the Brownstone Institute, the article completely misrepresented the results of the experiments, which don’t support and actually contradict the article’s claim, as we will explain below.
Also, while the article portrayed these data as concealed from the public, at least part of the results of these experiments have been publicly available since February 2021 on the websites of other regulatory agencies and in a study published by Pfizer and BioNTech in Reproductive Toxicology in August 2021, which was online since 28 May 2021[1].
Pfizer’s study in rats showed no adverse effects from the COVID-19 vaccine on fertility and offspring development
As part of the nonclinical evaluation of the mRNA COVID-19 vaccine, Pfizer conducted a reproduction toxicity study in rats to identify any potential adverse effects of the vaccine on fertility, reproduction, and development (pages 54 to 56, section six).
First, the researchers administered an intramuscular injection of a full adult human dose of the COVID-19 vaccine to female rats twice before mating and twice during gestation. According to the document, one full human dose is more than 300 times the dose per body weight in rats. Then, they compared several reproductive outcomes in these animals to a control group that hadn’t received the vaccine.
The article in the Brownstone Institute claimed that the results of this study showed a double rate of preimplantation loss and a significantly higher rate of fetal abnormalities in the vaccinated rats compared to the unvaccinated rats. However, the article completely misrepresented the data, which didn’t show any potential signs of concern.
The preimplantation loss estimates the percentage of fertilized eggs that don’t implant into the uterus, and therefore don’t advance to the next stages of pregnancy.
Each time an egg leaves the ovary (ovulation), a structure called corpus luteum forms inside the ovary and starts producing the hormone progesterone, which prepares the uterus for pregnancy (Figure 1). If pregnancy doesn’t occur, the corpus luteum dissolves. When an egg gets fertilized, it travels down the fallopian tube toward the uterus and starts dividing. Once it reaches the uterus, the formed cell cluster attaches itself to the lining of the uterus, a process called implantation (Figure 1).
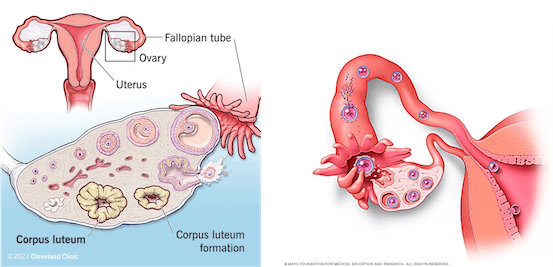
Figure 1. Illustration showing the ovarian cycle and the formation of the corpus luteum (left) and the stages of ovulation, fertilization, and implantation in the uterus (right). Source: Cleveland Clinic and Mayo Clinic.
Page 55 of Pfizer document shows variable values of preimplantation loss in female rats injected with three different formulations of the mRNA vaccine (BNT162b 1, 2, and 3), ranging from 4.8 to 9.8%. The rats injected with BNT162b2 showed a significant increase in preimplantation loss (9.8%) that is double that of the non-injected rats (4.1%) (Figure 2), which is the figure that article in the Brownstone Institute used to support its claim that COVID-19 vaccines harm fertility.
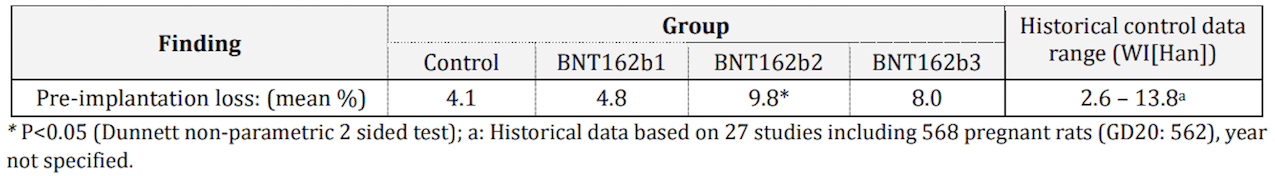
Figure 2. Preimplantation loss in rats that received one of the three mRNA COVID-19 vaccine formulations compared to unvaccinated rats and to historical control data on preimplantation loss from previous studies. Source: Australia’s Therapeutic Goods Administration.
While an increase in preimplantation loss might potentially indicate problems during egg transport, fertilization, or implantation, these values alone don’t necessarily imply that COVID-19 vaccines reduce fertility. To correctly evaluate these data, we need to consider them within the context of the other results of the study.
The authors of the study compared the values of preimplantation loss observed in vaccinated rats with historical control data from 27 studies, including 568 pregnant untreated rats (2.6 to 13.8%). These control data appear in the last column of Figure 2, and range from 2.6 to 13.8%. Therefore, all the values in vaccinated rats were well within the control range of preimplantation loss in these animals, suggesting that this apparent increase might simply reflect normal variability.
The rest of the analyses showed also no differences between vaccinated and unvaccinated rats in terms of implantation, embryo and fetal survival, birth, postnatal survival, and offspring growth, physical development, and neurofunctional development through the end of lactation.
The researchers also found no differences on maternal mortality and on the length, and regularity of the reproductive cycles. The only effect identified was a slight short-term decrease in body weight and food consumption following each dose injection, from which the animals completely recovered within one or two weeks.
These data, taken together, further suggest that the higher implantation loss observed in vaccinated rats isn’t the result of vaccine toxicity but simply normal biological variation in these animals.
The article’s claim that vaccinated rats had a “higher [fetal] abnormality rate in each of the 12 categories studied” compared to the control group is inaccurate as well. There were slight differences between the vaccinated and control group, but they weren’t statistically significant. This means that there is a high probability that the observed effects were due to chance (Figure 3). In the results of the Reproductive Toxicology study the authors stated that all the findings were “within the normal background incidence noted for this rat strain at the performing laboratory, and therefore were considered incidental”.
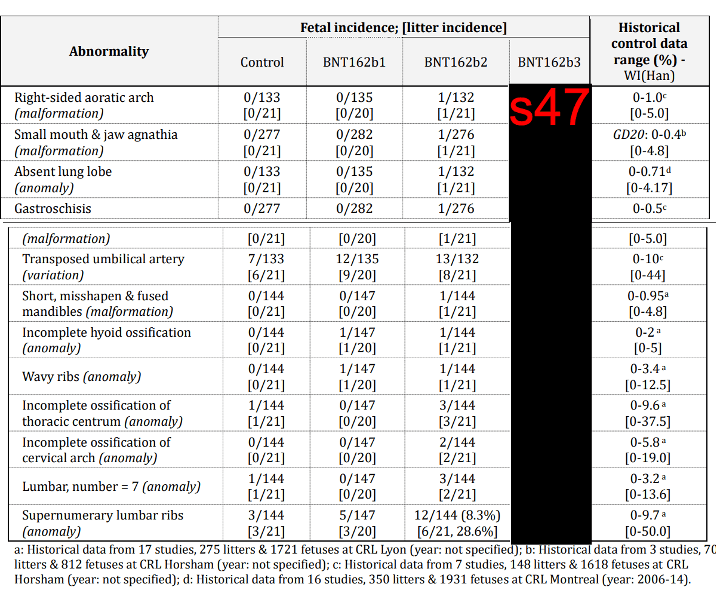
Figure 3. Fetal malformations in rats that received one of the three mRNA COVID-19 vaccine formulations compared to unvaccinated rats and to historical control data from previous studies. Data for BNT162b3 were redacted in the released document. Source: Australia’s Therapeutic Goods Administration.
Hence, contrary to the claim, the results from this study show that the COVID-19 vaccine was safe in pregnant rats, even though they received four injections of a full human dose, which is a few hundred times greater in rats when body weight is accounted for.
The majority of the lipid nanoparticles remain at the injection site; only tiny amounts reach the ovaries
The article in the Brownstone Institute claimed that the Pfizer document “demonstrated that the vaccine will travel throughout the body after injection, and is found not only at the injection site, but in all organs tested, with high concentration in the ovaries, liver, adrenal glands, and spleen”.
Earlier, on 24 March 2023, Australian Senator Gerard Rennick appeared on a podcast by John Campbell, making the same claim, along with other claims relating to the Pfizer document and the safety of the COVID-19 vaccine.
Health Feedback addressed this claim in an earlier review and found it to be inaccurate. The claim that the vaccine accumulates in the ovaries at high levels arises from a document that Pfizer submitted to Japan’s regulatory authority Pharmaceuticals and Medical Devices Agency (PMDA) as part of the COVID-19 vaccine authorization application.
Briefly, the document contained data on how lipid nanoparticles identical to the ones enclosing the mRNA in the Pfizer COVID-19 vaccine traveled and distributed to different body organs and tissues in rats injected with these particles.
But contrary to the claim, pages 5 and 6 of the document clearly showed that most lipid nanoparticles administered (52.6%) remained at the injection site, followed by the liver (18.1% at eight hours post-injection). In contrast, the amount of nanoparticles in the rest of the tissues didn’t exceed 1% of the administered dose. In the specific case of the ovaries, the peak concentration was only 0.095% of the administered dose at 48 hours post-injection.
As in the reproductive toxicity study, these animals received a much higher dose of lipid nanoparticles per kilogram of body weight than the dose that a person receives in a COVID-19 vaccine. Therefore, these results in rats don’t translate directly to humans.
Furthermore, there is no evidence suggesting that the small concentrations of lipid nanoparticles detected in the ovaries negatively affect ovarian function. On the contrary, large clinical studies showed no increased rate of placental defects, miscarriages, and negative pregnancy outcomes in vaccinated pregnant women[2,3].
Pregnant women are at a higher risk of developing severe COVID-19 and pregnancy complications resulting from it. But COVID-19 vaccines can reduce this risk by preventing severe illness[4]. For that reason, medical associations like the American College of Obstetricians and Gynecologists and public health agencies like the U.S. Centers for Disease Control and Prevention highly recommend that pregnant women get vaccinated against COVID-19.
REFERENCES
- 1 – Bowman et al. (2021) Lack of effects on female fertility and prenatal and postnatal offspring development in rats with BNT162b2, a mRNA-based COVID-19 vaccine. Reproductive Toxicology.
- 2 – Prasad et al.(2022) Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nature Communications.
- 3 – Goldshtein et al. (2022) Association of BNT162b2 COVID-19 Vaccination During Pregnancy With Neonatal and Early Infant Outcomes. JAMA Pediatrics.
- 4 – Villar et al. (2023) Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet.