- Health
Hundreds of millions of people worldwide have received at least one dose of COVID-19 vaccine; their safety and effectiveness are established; the vaccines aren’t experimental
Key takeaway
Data from clinical trials as well as post-marketing surveillance, conducted in millions of people, demonstrate that the COVID-19 vaccines have an excellent safety profile and are highly effective at keeping people out of hospitals and dying from COVID-19.
Reviewed content

Verdict:
Claim:
The COVID-19 vaccines are experimental
Verdict detail
Misleading: To date, the three COVID-19 vaccines used in the U.S. have either received full FDA approval (Pfizer-BioNTech) or Emergency Use Authorization (Moderna and Johnson & Johnson). Their safety and effectiveness in people were established in clinical trials before use in the public. Labeling them as “experimental” misrepresents our state of understanding of the vaccines.
Full Claim
"It’s a brand new virus and formula and the science is continually evolving but the White House wants us to keep injecting these experimental vaccines into our arms until they get it right and figure it out? NO!"
Review
Political commentator Tomi Lahren posted a tweet on 19 January 2022, claiming that the COVID-19 vaccines are experimental. The tweet received more than 1,100 engagements. A screenshot of her tweet was also published to Lahren’s Facebook page, where it received another 7,500 user engagements.
Claims that the COVID-19 vaccines are experimental and/or that vaccinated people are guinea pigs aren’t new. They circulated early in 2020, as this previous Health Feedback review reported. At that time, three COVID-19 vaccines (Pfizer-BioNTech, Moderna, Johnson & Johnson) were authorized for use under the U.S. Food and Drug Administration’s Emergency Use Authorization program. Since then, the Pfizer-BioNTech COVID-19 vaccine received FDA approval. Nevertheless, the claim that the vaccines are experimental proves to be popular, as multiple Health Feedback reviews about the same claim in viral social media posts by personalities such as another political commentator Candace Owens and chiropractor Benjamin Benulis, show.
The safety and effectiveness of COVID-19 vaccines were established in clinical trials before receiving EUA
The FDA describes the concept of an EUA as follows:
“Under an EUA, FDA may allow the use of unapproved medical products, or unapproved uses of approved medical products in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, including that there are no adequate, approved, and available alternatives.”
An EUA can only be issued if the government declares a public health emergency. This description illustrates that the EUA is specifically designed to address serious public health threats, such as that posed by the COVID-19 pandemic, when a swift reaction is needed.
More importantly, a vaccine candidate being considered for the EUA must have undergone clinical trials. Specifically, the FDA requires data from Phase 1, Phase 2, and Phase 3 clinical trials that demonstrate the safety and efficacy of the vaccine before an EUA can be issued. The data are then reviewed by a panel of experts in vaccines and infectious diseases who determine whether the benefits of the vaccine outweigh its risks.
The FDA made the data from the clinical trials for the Pfizer-BioNTech, Moderna, and Johnson & Johnson vaccines publicly available, which showed that the majority of observed adverse events are mild, short-lived, and characteristic of the body’s reaction to vaccination. They include redness and pain at the site of injection, fever or headache. Serious adverse events were extremely rare, with less than 1% of the volunteers experiencing such events. Significantly, such events occurred at the same proportion between the volunteers who received the vaccine and those who didn’t (control group). This strongly suggests that none of the serious adverse events observed during the trials were caused by the vaccine.
The results of the clinical trials for the RNA vaccines produced by Pfizer-BioNTech and Moderna, and established by Phase 2 and 3 clinical trials in tens of thousands of people, are published in scientific journals[1,2]. Phase 1 and 2 trials of the Johnson & Johnson vaccine were published in the New England Journal of Medicine, showing the vaccine to be safe[3], while an interim analysis of the Phase 3 trials, currently underway in tens of thousands of people, for the same vaccine also showed that the vaccine is safe and effective at protecting adults from severe disease.
There are also some who claimed or implied that testing for COVID-19 vaccines aren’t completed, because the relevant entries on ClinicalTrials.gov, a database of clinical trial records run by the U.S. Library of Medicine, list an estimated completion date in 2022 or 2023. This is a misunderstanding of what estimated completion dates listed in the database mean; a trial can achieve its set goals earlier than the projected completion date, and this is in fact the case for the Pfizer-BioNTech COVID-19 vaccine, which already received approval.
Hundreds of millions of people around the world have already received at least one dose of COVID-19 vaccine; their safety and effectiveness is well-established
It’s standard procedure for vaccines to continue to be monitored even after they are permitted for public health use. Post-marketing surveillance, which can be a part of clinical trials (Phase 4), allows regulatory agencies to monitor safety even after a drug or vaccine has reached the market. Such surveillance programs allow agencies to assess safety in an even larger population than that included in clinical trials. An example of post-marketing surveillance is the Vaccine Adverse Event Reporting System. The FDA outlined several other post-marketing surveillance programs here.
At the time of this review’s writing, more than 9.84 billion doses of COVID-19 vaccine have been administered worldwide, with the U.S. contributing a share of 530 million doses to that number. The U.S. Centers for Disease Control and Prevention COVID Data Tracker reported on 20 January 2022 that 209.8 million people in the U.S. are fully vaccinated, and that more than 80.1% of the U.S. population aged five and above have received at least one dose (see below).
![]()
Figure 1. The CDC’s COVID Data Tracker on COVID-19 vaccinations in the U.S. Data retrieved on 21 January 2022 from this webpage.
Based on data from clinical trials and post-marketing surveillance of millions of people around the world, we know that COVID-19 vaccines demonstrate a high level of safety. Nor are COVID-19 vaccines associated with an increased risk of pregnancy complications[4-7] or infertility[8], two other commonly propagated false claims about COVID-19 vaccines.
It’s true that researchers discovered certain serious side effects associated with COVID-19 vaccines, such as blood clots and heart inflammation. However, studies showed that these complications are much more likely from getting COVID-19 than from getting the vaccines[9, 10], as reported by the BBC and National Geographic. Consequently, the benefits of getting the vaccine outweigh the risks[11, 12].
Apart from its established safety, we also have plenty of evidence in millions of people in different countries establishing that the vaccines are highly effective at keeping people out of hospitals and dying from COVID-19, as data presented by Our World in Data illustrates below.
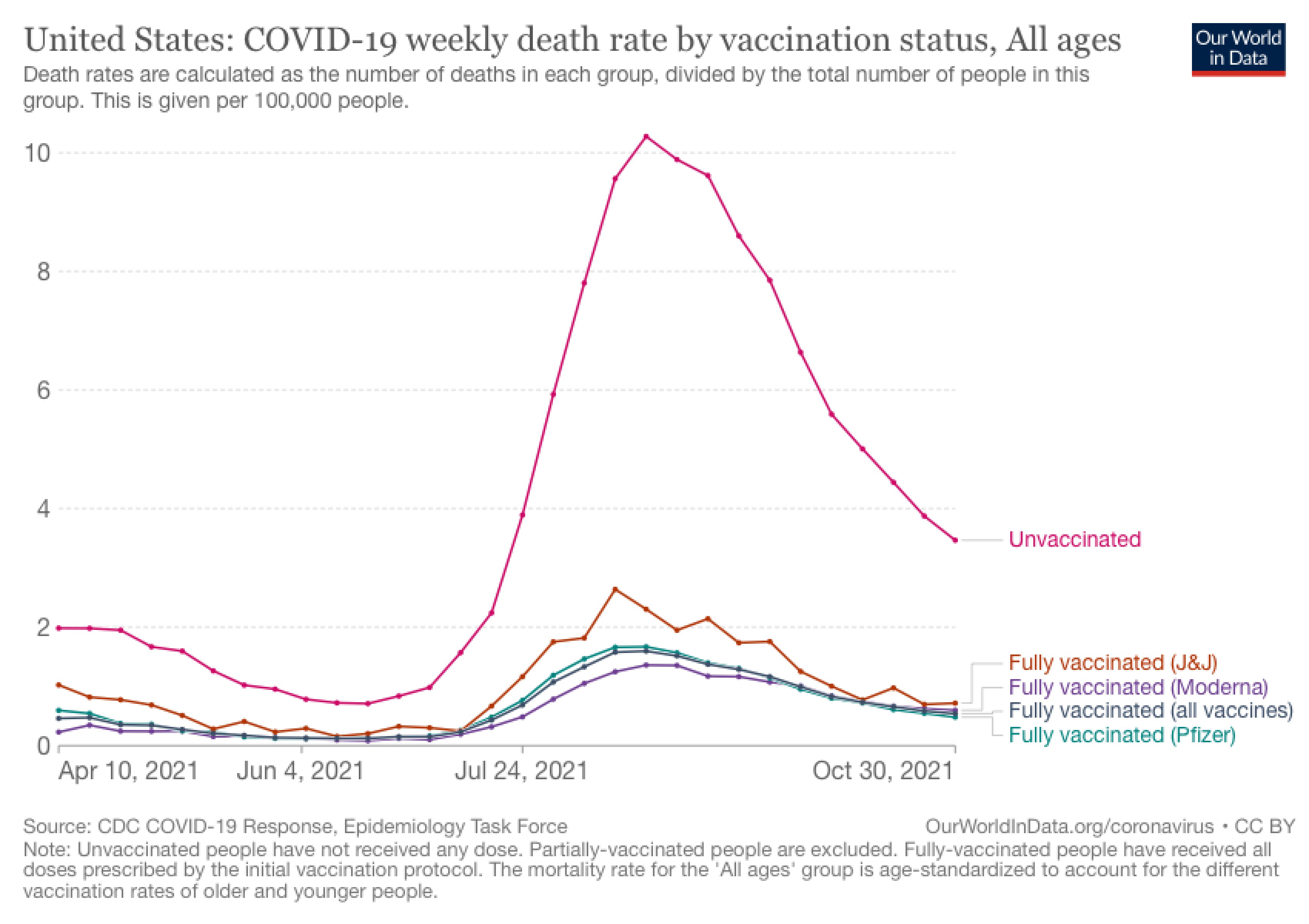
Figure 2. The U.S. COVID-19 weekly mortality rate (per 100,000 people) based on vaccination status. Chart from Our World in Data. The number of fully vaccinated people in the U.S. is estimated to be about 209.1 million.
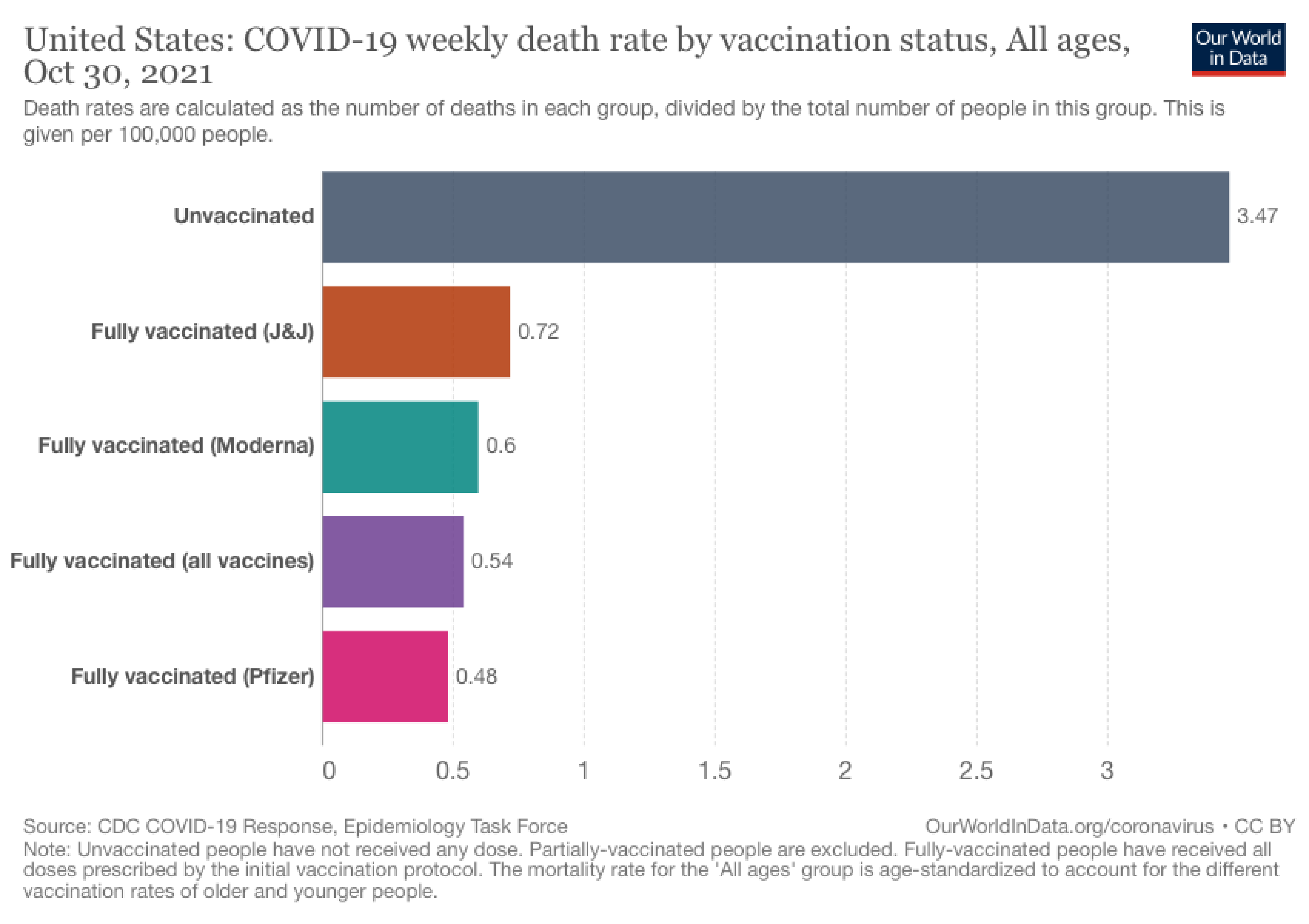
Figure 3. The U.S. COVID-19 weekly mortality rate (per 100,000 people) based on vaccination status, represented as a bar chart. Chart from Our World in Data.
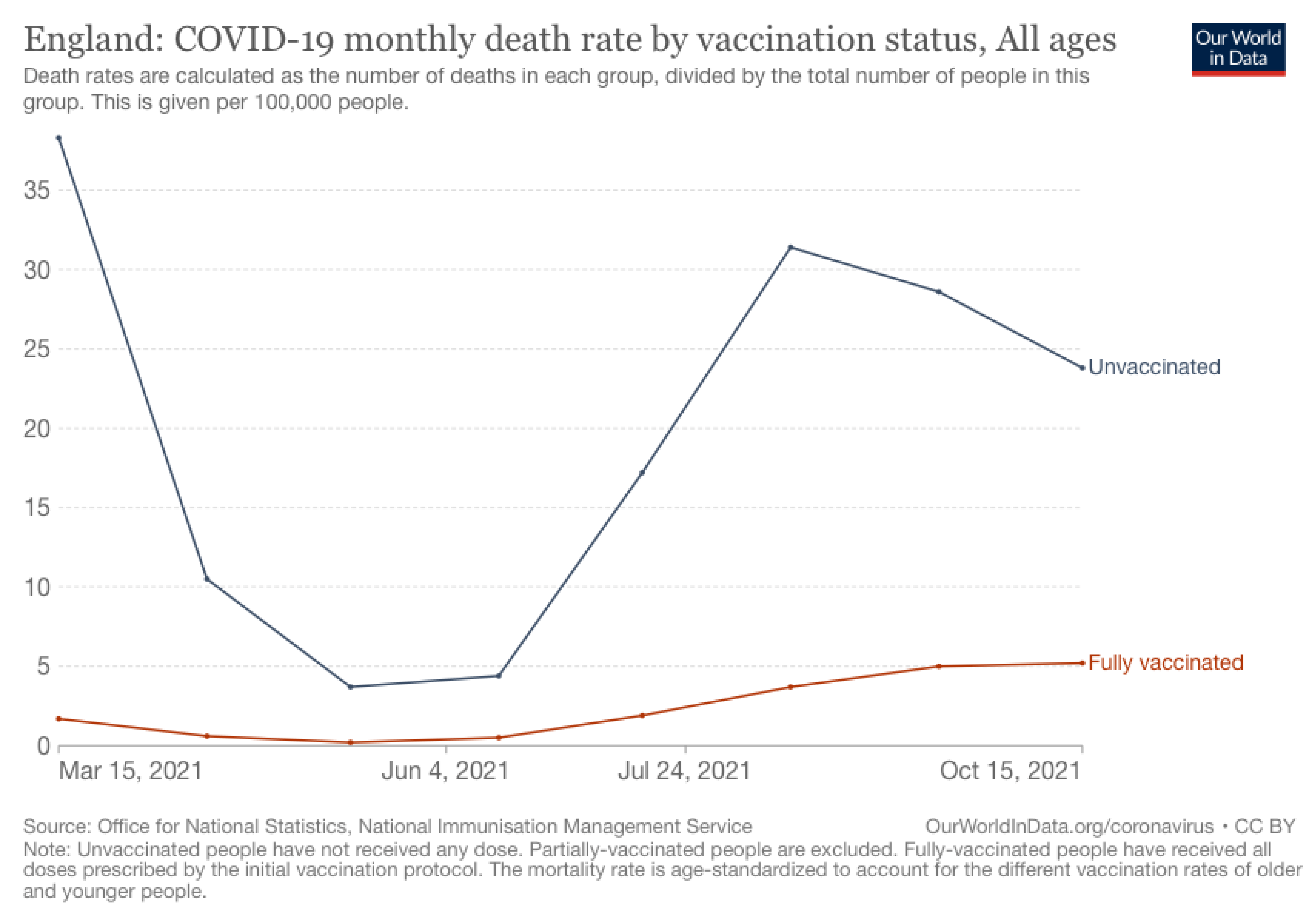
Figure 4. England’s COVID-19 weekly mortality rate (per 100,000 people) based on vaccination status. Chart from Our World in Data. The number of fully vaccinated people in the U.K. is estimated to be about 48.1 million.
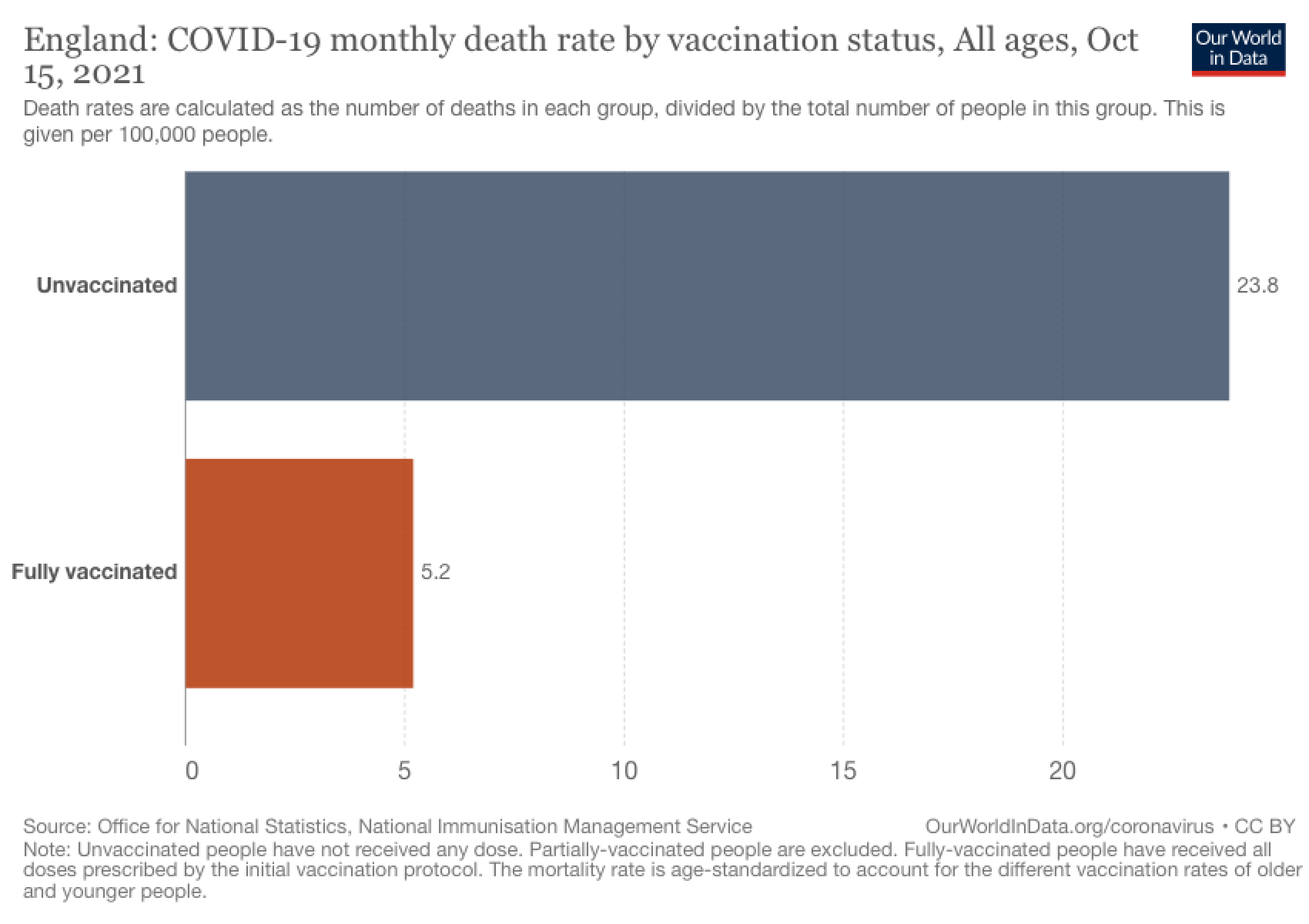
Figure 5. England’s COVID-19 monthly mortality rate (per 100,000 people) based on vaccination status, represented as a bar chart. Chart from Our World in Data.
REFERENCES
- 1 – Polack et al. (2020) Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine.
- 2 – Baden et al. (2021) Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine.
- 3 – Sadoff et al. (2021) Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. New England Journal of Medicine.
- 4 – Stock et al. (2022) SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nature Medicine.
- 5 – Lipkind et al. (2022) Receipt of COVID-19 Vaccine During Pregnancy and Preterm or Small-for-Gestational-Age at Birth — Eight Integrated Health Care Organizations, United States, December 15, 2020–July 22, 2021. Mortality and Morbidity Weekly Report.
- 6 – Wainstock et al. (2021) Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine.
- 7 – Blakeway et al. (2021) COVID-19 vaccination during pregnancy: coverage and safety. American Journal of Obstetrics and Gynecology.
- 8 – Wesselink et al. (2022) A prospective cohort study of COVID-19 vaccination, SARS-CoV-2 infection, and fertility. American Journal of Epidemiology.
- 9 – Boehmer et al. (2021) Association Between COVID-19 and Myocarditis Using Hospital-Based Administrative Data — United States, March 2020–January 2021. Mortality and Morbidity Weekly Report.
- 10 – Hippisley-Cox et al. (2021) Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ.
- 11 – Gargano et al. (2021) Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices — United States, June 2021. Mortality and Morbidity Weekly Report.
- 12 – Bozkurt et al. (2021) Myocarditis With COVID-19 mRNA Vaccines. Circulation.